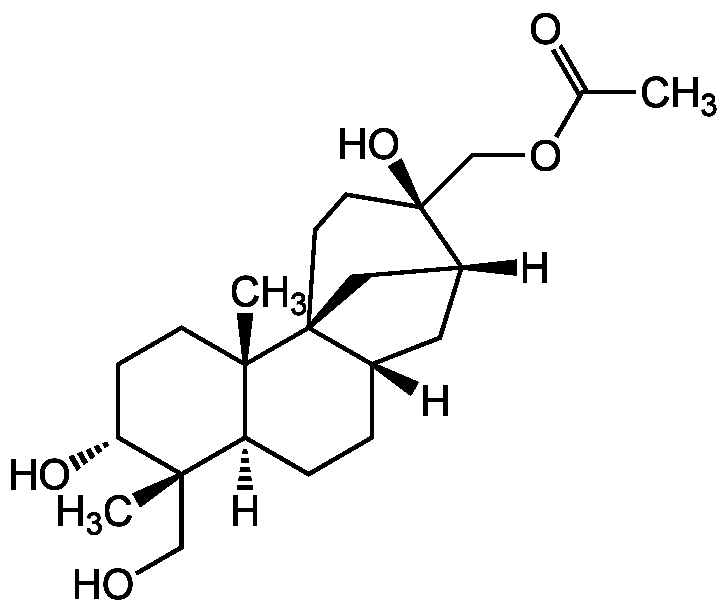
Aphidicolin 17-acetate
| Code | Size | Price |
|---|
| BVT-0337-C250 | 250 ug | £105.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
+4°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
17-O-Acetylaphidicolin; Aphidicolin 17-monoacetate
Appearance:
White to off-white solid.
CAS:
51103-57-2
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H319
InChi:
InChI=1S/C22H36O5/c1-14(24)27-13-22(26)9-8-21-11-16(22)10-15(21)4-5-17-19(2,12-23)18(25)6-7-20(17,21)3/h15-18,23,25-26H,4-13H2,1-3H3/t15-,16+,17-,18+,19-,20-,21-,22-/m0/s1
InChiKey:
GAPINCSXTLCIPV-BORIEPGUSA-N
Long Description:
Chemical. CAS: 51103-57-2. Formula: C22H36O5. MW: 380.5. Isolated from Phoma sp. BS 7210. Phytotoxin (inhibits root growth of seeding). Specific inhibitor of eukaryotic DNA polymerase alpha and DNA synthesis (competition with dCTP).
MDL:
N/A
Molecular Formula:
C22H36O5
Molecular Weight:
380.5
Package Type:
Plastic Vial
Precautions:
P280, P305, P351, P338
Product Description:
Phytotoxin (inhibits root growth of seeding). Specific inhibitor of eukaryotic DNA polymerase alpha and DNA synthesis (competition with dCTP).
Purity:
>96% (HPLC, 1H-NMR)
Signal Word:
Warning
SMILES:
[H][C@@]12C[C@]3([H])CC[C@@]4([H])[C@](C)(CO)[C@H](O)CC[C@]4(C)[C@]3(C1)CC[C@]2(O)COC(C)=O
Solubility Chemicals:
Soluble in DMSO or methanol.
Source / Host:
Isolated from Phoma sp. BS 7210.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Specific inhibitors of eukaryotic DNA synthesis and DNA polymerase alpha, 3-deoxyaphidicolin and aphidicolin-17-monoacetate: T. Haraguchi, et al.; Nucleic Acids Res. 11, 1197 (1983) | 3-Deoxyaphidicolin and aphidicolin analogues as phytotoxin from Phoma betae: A. Ichihara, et al.; Agric. Biol. Chem. 48, 1687 (1984) | Chemical modification of aphidicolin and the inhibitory effects of its derivatives on DNA polymerase alpha in vitro: S. Hiranuma, et al.; Chem. Pharm. Bull. 35, 1641 (1987) | Inhibition of DNA polymerase alpha by aphidicolin derivatives: L. Arabshahi, et al.; Nucleic Acids Res. 16, 5107 (1988) | Aphidicolin synthetic studies: a stereocontrolled end game: C. Rizzo, et al.; J. Chem. Soc. 5, 969 (1991) | Metabolites from Phoma sp. 7210, associated with Aizoon canariense: J. Dai, et al.; Nat. Prod. Comm. 5, 1175 (2010)