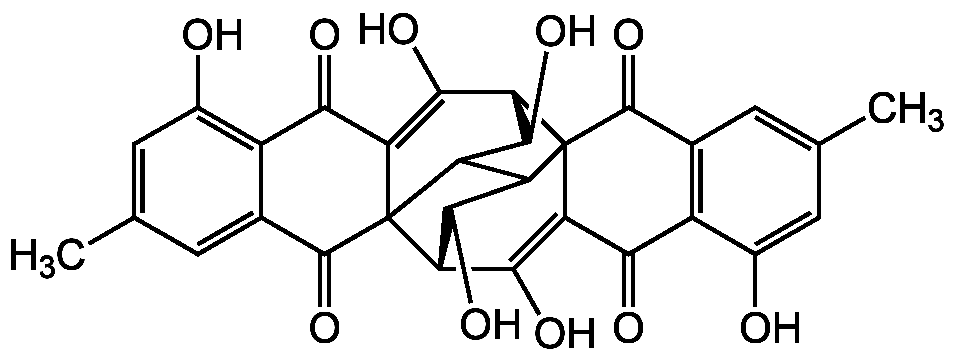
Rugulosin
| Code | Size | Price |
|---|
| BVT-0444-M005 | 5 mg | £365.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
+4°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(+)-Rugulosin; NSC 160880; NSC 249990; Radicalisin
Appearance:
Yellow solid.
CAS:
23537-16-8
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light when in solution.
Hazards:
H302, H312, H332
InChi:
InChI=1S/C30H22O10/c1-7-3-9-13(11(31)5-7)21(33)17-25(37)20-23(35)15-16-24(36)19(29(15,17)27(9)39)26(38)18-22(34)14-10(28(40)30(16,18)20)4-8(2)6-12(14)32/h3-6,15-16,19-20,23-24,31-36H,1-2H3
InChiKey:
QFDPVUTXKUGISP-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 23537-16-8. Formula: C30H22O10. MW: 542.5. Isolated from Penicillium sp. Mycotoxin. Antibiotic (Streptococcus, Corynebacterium, MRSA). Antiviral, HIV-1 integrase inhibitor. DNA replication, transcription and repair inhibitor (carcinogenic activity). RNA polymerase and ribonuclease H inhibitor. Insecticidal activity. Inhibitor of N-Hsp90.
MDL:
MFCD01684610
Molecular Formula:
C30H22O10
Molecular Weight:
542.5
Package Type:
Plastic Vial
Precautions:
P261, P301, P312, P302, P352, P304, P340
Product Description:
Mycotoxin. Antibiotic (Streptococcus, Corynebacterium, MRSA). Antiviral, HIV-1 integrase inhibitor. DNA replication, transcription and repair inhibitor (carcinogenic activity). RNA polymerase and ribonuclease H inhibitor. Insecticidal activity. Inhibitor of N-Hsp90.
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
CC1=CC2=C(C(O)=C1)C(=O)C1=C(O)[C@H]3C(O)C4C5C(O)[C@H](C(O)=C6C(=O)C7=C(O)C=C(C)C=C7C(=O)C356)C14C2=O
Solubility Chemicals:
Soluble in DMSO, acetone, ethanol or methanol.
Source / Host:
Isolated from Penicillium sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
Studies in the biochemistry of micro-organisms. 95. Rugulosin, a crystalline colouring matter of Penicillium rugulosum Thom: J. Breen, et al.; Biochem. J. 60, 618 (1955) | Inhibition of phage growth by an antibiotic rugulosin isolated from Myrothecium verucaria. I. Properties of the anti-phage effect: S. Nakamura, et al.; Jpn. J. Microbiol. 15, 113 (1971) | Toxicological approach to (+) rugulosin, an anthraquinoid mycotoxin of Penicillium rugulosum Thom: Y. Ueno, et al.; Jpn. J. Exp. Med. 41, 177 (1971) | Specific and non-specific interactions of two carcinogenic mycotoxins, luteoskyrin and rugulosin with nucleic acids: J.C. Bouhet, et al.; Ann. Nutr. Aliment. 31, 811 (1977) | Mutagenicity and antibacterial activity of mycotoxins produced by Penicillium islandicum Sopp and Penicillium rugulosum: A.A. Stark, et al.; J. Environ. Pathol. Toxicol. 2, 313 (1978) | Inhibitory effects of carcinogenic mycotoxins on deoxyribonucleic acid-dependent ribonucleic acid polymerase and ribonuclease H: F. Tashiro, et al.; Appl. Environ. Microbiol. 38, 191 (1979) | Cytotoxicity against insect cells of entomopathogenic fungi of the genera Hypocrella (anamorph Aschersonia): possible agents for biological control: P. Watts, et al.; Mycol. Res. 107, 581 (2003) | Isolation, structure, and HIV-1-integrase inhibitory activity of structurally diverse fungal metabolites: S.B. Singh, et al.; J. Ind. Microbiol. Biotechnol. 30, 721 (2003) | New rugulosins, anti-MRSA antibiotics, produced by Penicillium radicum FKI-3765-2: H. Yamazaki, et al.; Org. Lett. 12, 1572 (2010) | Inhibition and binding of Rugulosin with N-Hsp90: J.-J.Chen, et al.; Gaodeng Xuexiao Huaxue Xuebao 32, 88 (2011) | Antibiotically active metabolites from Talaromyces wortmannii, an endophyte of Aloe vera: R. Bara, et al.; J. Antibiot. 66, 491 (2013) | Distribution of the foliar fungal endophyte Phialocephala scopiformis and its toxin in the crown of a mature white spruce tree as revealed by chemical and qPCR analyses: S. L. Frasz, et al.; Can. J. Forest Res. 44,1138 (2014) | Comparison of cytotoxic extracts from fruiting bodies, infected insects and cultured mycelia of Cordyceps formosana: R.-L. Lu, et al.; Food Chem. 145, 1066 (2014)