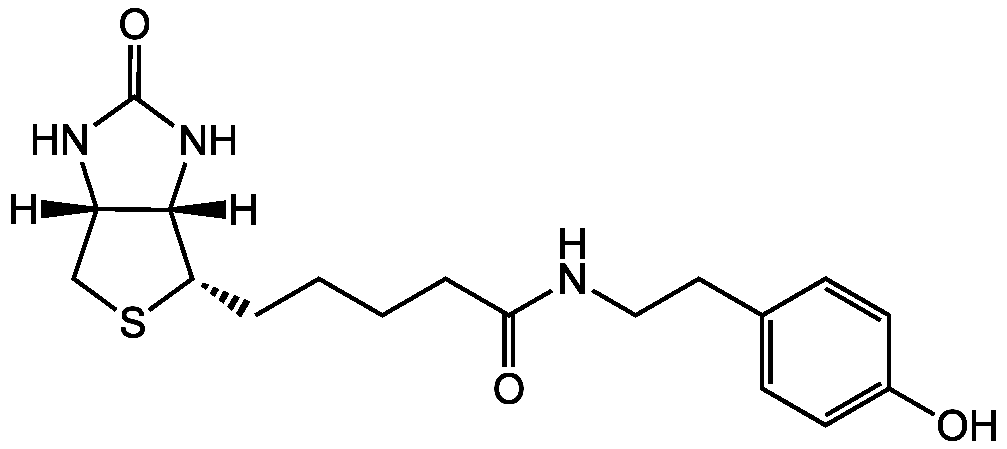
Biotinyl tyramide
| Code | Size | Price |
|---|
| CDX-B0270-M100 | 100 mg | £169.00 |
Quantity:
| CDX-B0270-M500 | 500 mg | £645.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Biotin-Phenol; BP; N-(4-Hydroxyphenethyl)-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamide
Appearance:
White to light pink solid.
CAS:
41994-02-9
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C18H25N3O3S/c22-13-7-5-12(6-8-13)9-10-19-16(23)4-2-1-3-15-17-14(11-25-15)20-18(24)21-17/h5-8,14-15,17,22H,1-4,9-11H2,(H,19,23)(H2,20,21,24)/t14-,15-,17-/m0/s1
InChiKey:
VZWXNOBHWODXCW-ZOBUZTSGSA-N
Long Description:
Chemical. CAS: 41994-02-9. Formula: C18H25N3O3S. MW: 363.5. Synthetic. Biotin derivative. Substrate of the horseradish peroxidase enzyme and used as a reagent to amplify immunohistochemical signals. It is based on the HRP-catalyzed deposition of tyramide conjugates (such as biotinyl-tyramide) on a solid phase. Subsequent reaction with streptavidin fluorophore results in the localization of the fluorophore at the site of tyramide deposition. This fluorescence-based tyramide signal amplification (TSA) has been widely used in immunohistochemistry, immunohistochemistry, immunoelectron microscopy, fluorescent in situ hybridization (FISH) and fluorescence ELISA. The TSA method has been reported to increase the detection sensitivity up to 100-fold as compared with conventional avidin?biotinylated enzyme complex procedures. It can be used together with both chromogenic and fluorescence visualization methods. It can be added to any other standard IHC protocol and reduces the use of other reagents; improves signal to noise by reducing the titer of other reagents in the assay protocol and enables multi-target detection in both IHC and (F)ISH applications.
MDL:
MFCD27953026
Molecular Formula:
C18H25N3O3S
Molecular Weight:
363.5
Package Type:
Vial
Product Description:
Biotin derivative. Substrate of the horseradish peroxidase enzyme and used as a reagent to amplify immunohistochemical signals. It is based on the HRP-catalyzed deposition of tyramide conjugates (such as biotinyl-tyramide) on a solid phase. Subsequent reaction with streptavidin fluorophore results in the localization of the fluorophore at the site of tyramide deposition. This fluorescence-based tyramide signal amplification (TSA) has been widely used in immunohistochemistry, immunoelectron microscopy, fluorescent in situ hybridization (FISH) and fluorescence ELISA. The TSA method has been reported to increase the detection sensitivity up to 100-fold as compared with conventional avidin?biotinylated enzyme complex procedures. It can be used together with both chromogenic and fluorescence visualization methods. It can be added to any other standard IHC protocol and reduces the use of other reagents; improves signal to noise by reducing the titer of other reagents in the assay protocol and enables multi-target detection in both IHC and (F)ISH applications.
Purity:
>97% (HPLC)
SMILES:
[H][C@]12CS[C@@H](CCCCC(=O)NCCC3=CC=C(O)C=C3)[C@@]1([H])NC(=O)N2
Solubility Chemicals:
Soluble in DMSO or acetonitrile.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) B. Hunyady, et al.; J. Histochem. Cytochem. 44, 1353 (1996) | (2) G. Mayer, et al.; J. Histochem. Cytochem. 45, 1449 (1997) | (3) J.A. McKay, et al.; Mol. Path. 50, 322 (1997) | (4) M.F. Evans, et al.; Mod. Pathol. 15, 1339 (2002) | (5) Q. Chenac, et al.; Anal. Lett. 45, 219 (2012) | (6) H. Gong, et al.; Anal. Bioch. 426, 27 (2012) | (7) E. Draberova, et al.; J. Immunol. Methods 395, 63 (2013) | RANKL deletion in periodontal ligament and bone lining cells blocks orthodontic tooth movement: C.Y. Yang, et al.; Int. J. Oral Sci. 10, 3 (2018) | Mapping the mammalian ribosome quality control complex interactome using proximity labeling approaches: N. Zuzow, et al.; MBoC, ahead of print (2018) | Identification of novel dense-granule proteins in Toxoplasma gondii by two proximity-based biotinylation approaches: M. Pan, et al.; J. Proteom. Res. in press (2018) | Proximity Labeling To Map Host-Pathogen Interactions at the Membrane of a Bacterium-Containing Vacuole in Chlamydia trachomatis-Infected Human Cells: M.G. Olson, et al.; Infect. Immun. 87, e00537 (2019) | Coupling APEX labeling to imaging mass spectrometry of single organelles reveals heterogeneity in lysosomal protein turnover: D.P. Narendra, et al.; J. Cell Biol. ahead of print, (2019) | Spatially resolved cell polarity proteomics of a human epiblast model: S. Wang, et al.; Sci. Adv. 7, (2021) | Mammalian hybrid pre-autophagosomal structure HyPAS generates autophagosomes: S. Kumar, et al.; Cell in press, (2021)
Related Products
| Product Name | Product Code | Supplier | N-(2-Aminoethyl)biotinamide hydrochloride | CDX-A0191 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biotin-(5-fluorescein)-conjugate | CDX-B0078 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D-Biotin | CDX-B0148 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||