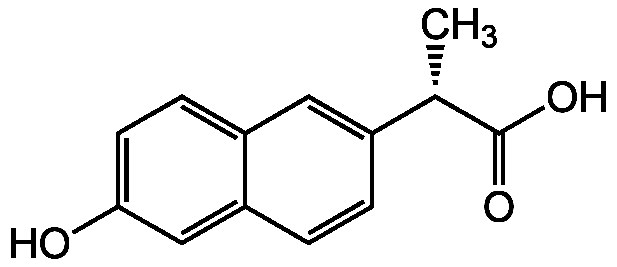
(S)-O-Desmethylnaproxen
| Code | Size | Price |
|---|
| CDX-D0390-M010 | 10 mg | £157.00 |
Quantity:
| CDX-D0390-M050 | 50 mg | £596.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(S)-6-Hydroxy-alpha-methyl-2-naphthaleneacetic acid; (S)-6-O-Desmethylnaproxen
Appearance:
White to off-white solid.
CAS:
52079-10-4
Class:
9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS09
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H302, H317, H400
InChi:
InChI=1S/C13H12O3/c1-8(13(15)16)9-2-3-11-7-12(14)5-4-10(11)6-9/h2-8,14H,1H3,(H,15,16)/t8-/m0/s1
InChiKey:
XWJUDDGELKXYNO-QMMMGPOBSA-N
Long Description:
Chemical. CAS: 52079-10-4. Formula: C13H12O3. MW: 216.2. Synthetic. Major naproxen demethylation metabolite. Naproxen demethylation is mediated primarily by CYP2C9. Can be used for assaying naproxen metabolites.
MDL:
MFCD00869765
Molecular Formula:
C13H12O3
Molecular Weight:
216.2
Package Type:
Vial
PG:
III
Precautions:
P273, P280
Product Description:
Major naproxen demethylation metabolite. Naproxen demethylation is mediated primarily by CYP2C9. Can be used for assaying naproxen metabolites.
Purity:
>97% (HPLC)
Signal word:
Warning
SMILES:
C[C@H](C(O)=O)C1=CC2=CC=C(O)C=C2C=C1
Solubility Chemicals:
Soluble in chloroform.
Source / Host:
Synthetic.
Transportation:
Excepted Quantity
UN Nummer:
UN 3077
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) C.H. Kiang, et al.; Drug Metab. Dispos. 17, 43 (1989) | (2) A. Rettie, et al.; Chem. Res. Toxicol. 5, 54 (1992) | (3) T.B. Vree, et al.; Br. J. Clin. Pharmacol. 35, 467 (1993) | (4) T.B. Vree, et al.; Biopharm. Drug Dispos. 14, 491 (1993) | (5) P. Bonnabry, et al.; Eur. J. Clin. Pharmacol. 49, 305 (1996) | (6) J.O. Miners, et al.; Biochem. Pharmacol. 51, 1003 (1996) | (7) T.S. Tracy, et al.; Eur. J. Clin. Pharmacol. 52, 293 (1997) | (8) S. Rao, et al.; J. Med. Chem. 43, 2789 (2000) | (9) L. Wei, et al.; Mol. Pharmacol. 72, 1280 (2007) | (10) K.C. Duggan, et al.; J. Biol. Chem. 285, 34950 (2010)