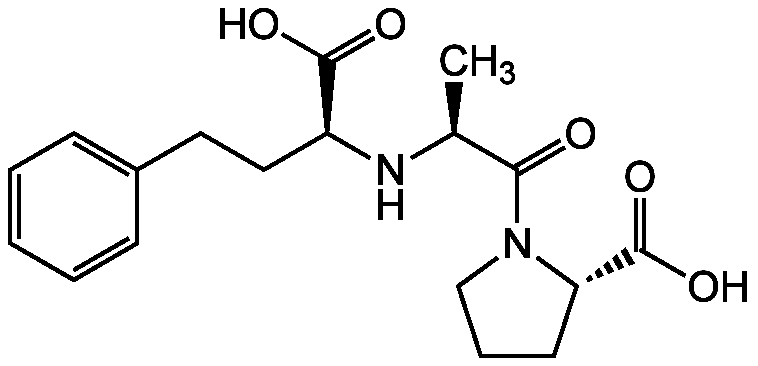
Enalaprilat
| Code | Size | Price |
|---|
| CDX-E0090-M050 | 50 mg | £157.00 |
Quantity:
| CDX-E0090-M250 | 250 mg | £450.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
EAL; Enalapril acid; MK-422
Appearance:
White solid.
CAS:
76420-72-9
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1
InChiKey:
LZFZMUMEGBBDTC-QEJZJMRPSA-N
Long Description:
Chemical. CAS: 76420-72-9. Formula: C18H24N2O5. MW: 348.4. Synthetic. Active metabolite enalapril. Nonsulfhydryl angiotensin-converting enzyme (ACE) inhibitor. Shown to improve cardiac preservation via bradykinin receptor and PKC without affecting ATP metabolism. Has some antioxidant activity, which does not include the PKC-NADPH oxidase pathway. Increases PPARbeta/delta expression, without influence on PPARalpha and PPARgamma.
MDL:
MFCD00865786
Molecular Formula:
C18H24N2O5
Molecular Weight:
348.4
Package Type:
Vial
Product Description:
Active metabolite enalapril. Nonsulfhydryl angiotensin-converting enzyme (ACE) inhibitor. Shown to improve cardiac preservation via bradykinin receptor and PKC without affecting ATP metabolism. Has some antioxidant activity, which does not include the PKC-NADPH oxidase pathway. Increases PPARbeta/delta expression, without influence on PPARalpha and PPARgamma.
Purity:
>99% (HPLC)
SMILES:
C[C@H](N[C@@H](CCC1=CC=CC=C1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O
Solubility Chemicals:
Slightly soluble in water.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) D.J. DiPette, et al.; Clin. Pharmacol. Ther. 38, 199 (1985) | (2) J.G. Kelly & K. O'Malley; Clin. Pharmacokinet. 19, 177 (1990) | (3) X. Yang, et al.; J. Mol. Cell Cardiol. 28, 1445 (1996) | (4) R. Lupi, et al.; Eur. J. Endocrinol. 154, 355 (2006) | (5) M. Yu, et al.; Molecules 17, 2738 (2012) | (6) H. Cernecka, et al.; Eur. J. Pharmacol. 714, 472 (2013)