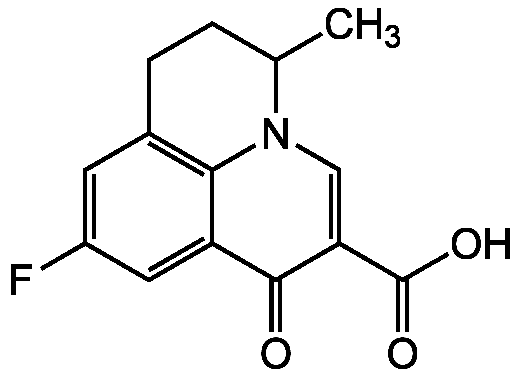
Flumequine
| Code | Size | Price |
|---|
| CDX-F0079-G005 | 5 g | £96.00 |
Quantity:
| CDX-F0079-G025 | 25 g | £353.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
9-Fluoro-5-methyl-1-oxo-1,5,6,7-tetrahydropyrido[3,2,1-ij]quinoline-2-carboxylic acid
Appearance:
White to off-white powder.
CAS:
42835-25-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C14H12FNO3/c1-7-2-3-8-4-9(15)5-10-12(8)16(7)6-11(13(10)17)14(18)19/h4-7H,2-3H2,1H3,(H,18,19)
InChiKey:
DPSPPJIUMHPXMA-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 42835-25-6. Formula: C14H12FNO3. MW: 261.3. Synthetic. Flumequine is a fluoroquinolone synthetic chemotherapeutic antibiotic used to treat bacterial infections. Targets primarily gram negative bacteria, especially those which cause enteric infections in animals. It is used to study processes that affect mammalian chromosome and DNA unwinding at the level of gyrase/topoisomerases. It inhibits topoisomerases, which are needed for the transcription and replication of bacterial DNA. The inhibition of the topoisomerases results in strand breakage of the bacterial chromosome, supercoiling and resealing. Therefore, DNA replication and transcription is inhibited. It was also used to study hepatocarcinogenicity and DNA damage in mice and the mechanisms of quinolone resistance.
MDL:
MFCD00079298
Molecular Formula:
C14H12FNO3
Molecular Weight:
261.3
Package Type:
Vial
Product Description:
Flumequine is a fluoroquinolone synthetic chemotherapeutic antibiotic used to treat bacterial infections. Targets primarily gram negative bacteria, especially those which cause enteric infections in animals. It is used to study processes that affect mammalian chromosome and DNA unwinding at the level of gyrase/topoisomerases. It inhibits topoisomerases, which are needed for the transcription and replication of bacterial DNA. The inhibition of the topoisomerases results in strand breakage of the bacterial chromosome, supercoiling and resealing. Therefore, DNA replication and transcription is inhibited. It was also used to study hepatocarcinogenicity and DNA damage in mice and the mechanisms of quinolone resistance.
Purity:
>97% (HPLC)
SMILES:
CC1CCC2=C3N1C=C(C(O)=O)C(=O)C3=CC(F)=C2
Solubility Chemicals:
Soluble in toluene.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) G. Stilwell, et al.; Antimicrob. Agents Chemother. 7, 483 (1975) | (2) S.R. Rohlfing, et al.; J. Antimicrob. Chemother. 3, 615 (1977) | (3) M. Yoshida, et al.; Cancer Lett. 141, 99 (1999) | (4) A. Ruiz-Garcia, et al.; Eur. J. Pharm. Biopharm. 48, 253 (1999) | (5) Y. Kashida, et al.; Toxicol. Sci. 69, 317 (2002) | (6) Y. Kuroiwa, et al.; Arch. Toxicol. 81, 63 (2007)
Related Products
| Product Name | Product Code | Supplier | Clarithromycin N-oxide | CDX-C0310 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Josamycin | CDX-J0001 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2-Methyl-5-nitroimidazole-1-acetic acid | CDX-M0091 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||