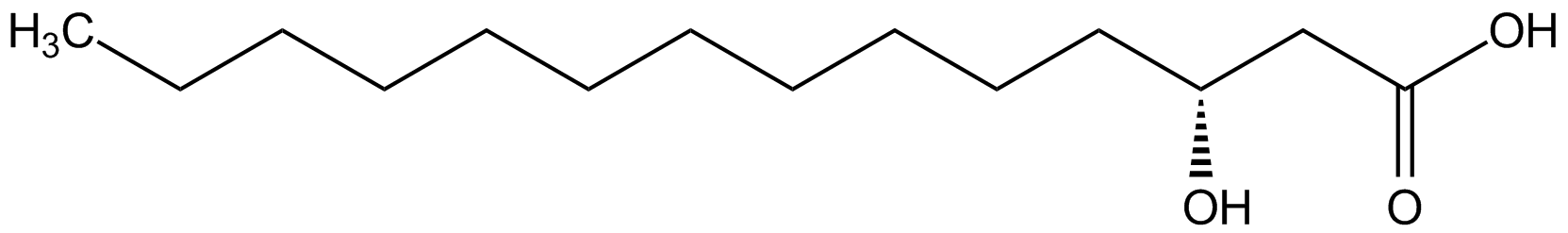
(R)-3-Hydroxymyristic acid
| Code | Size | Price |
|---|
| CDX-H0114-M250 | 250 mg | £364.00 |
Quantity:
| CDX-H0114-G001 | 1 g | £1,073.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
3-HTA; 3-Hydroxytetradecanoic acid
Appearance:
White powder.
CAS:
28715-21-1
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C14H28O3/c1-2-3-4-5-6-7-8-9-10-11-13(15)12-14(16)17/h13,15H,2-12H2,1H3,(H,16,17)/t13-/m1/s1
InChiKey:
ATRNZOYKSNPPBF-CYBMUJFWSA-N
Long Description:
Chemical. CAS: 28715-21-1. Formula: C14H28O3. MW: 244.4. Synthetic. Hydroxy fatty acid used to study its role in biological processes such as oxidative stress, inflammation and insulin resistance. Used in endotoxin and lipid A research, since it is one of two major components of bacterial Lipid A. Shows antifungal activity. Compound could be used as analytical reference in assaying food samples.
MDL:
MFCD00211333
Molecular Formula:
C14H28O3
Molecular Weight:
244.4
Package Type:
Vial
Product Description:
Hydroxy fatty acid used to study its role in biological processes such as oxidative stress, inflammation and insulin resistance. Used in endotoxin and lipid A research, since it is one of two major components of bacterial Lipid A. Shows antifungal activity. Compound could be used as analytical reference in assaying food samples.
Purity:
>98% (NMR)
SMILES:
CCCCCCCCCCC[C@@H](O)CC(O)=O
Solubility Chemicals:
Soluble in chloroform, dichloromethane, ether or ethyl acetate.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) K.W. Broady, et al.; Eur J Biochem 115, 463-468 (1981) | (2) K. Tanamoto; Adv. Exp. Med. Biol. 256, 203 (1990) | (3) K. Fukase, et al.; Tetrahed. Lett. 36, 7455 (1995) | (4) J. Sjoegren, et al.; Appl. Environ. Microbiol. 69, 7554 (2003) | (5) A.S. Soydan, et al.; Med. Inflamm. 2006, 64980 (2006) | (6) R. Jenske, et al.; J. Agric. Food Chem. 56, 11578 (2008) | (7) D. Six, et al.; Biochem. 47, 8623 (2008) | (8) A.M. Tonin, et al.; Neurochem. Int. 56, 930 (2010)