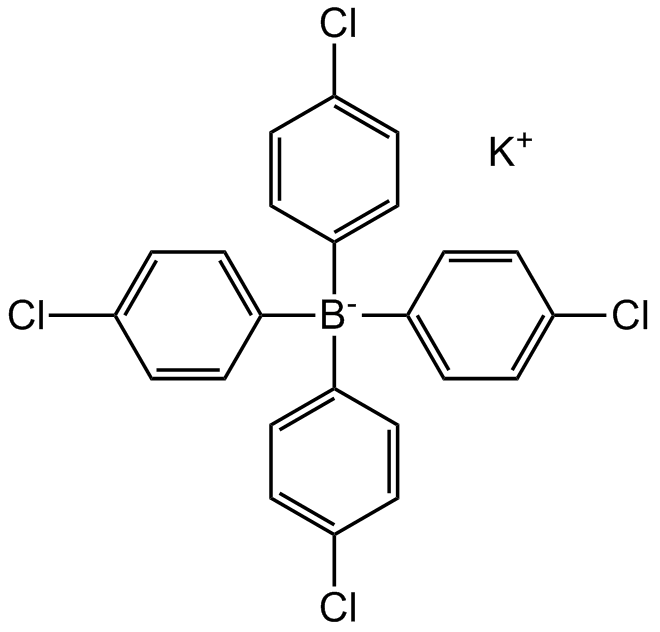
Potassium tetrakis(4-chlorophenyl)borate
| Code | Size | Price |
|---|
| CDX-P0134-G001 | 1 g | £114.00 |
Quantity:
| CDX-P0134-G005 | 5 g | £346.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Tetrakis(4-chlorophenyl)boron potassium; K-TCPB
Appearance:
White to off-white powder.
CAS:
14680-77-4
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C24H16BCl4.K/c26-21-9-1-17(2-10-21)25(18-3-11-22(27)12-4-18,19-5-13-23(28)14-6-19)20-7-15-24(29)16-8-20;/h1-16H;/q-1;+1
InChiKey:
SAGICZRAKJSWLD-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 14680-77-4. Formula: C24H16BCl4K. MW: 496.1. Synthetic. Lipophilic anionic additive, used together with selective ionophores. The ionophore is the ion carrier which reversibly binds ions and transports them across the hydrophobic membrane. The ionic additive K-TCPB, catalyzes the ion-exchange process at the sample/membrane interface.
MDL:
MFCD00043105
Molecular Formula:
C24H16BCl4K
Molecular Weight:
496.1
Package Type:
Vial
Precautions:
P261, P305, P351, P338
Product Description:
Lipophilic anionic additive, used together with selective ionophores. The ionophore is the ion carrier which reversibly binds ions and transports them across the hydrophobic membrane. The ionic additive K-TCPB, catalyzes the ion-exchange process at the sample/membrane interface.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
[K+].ClC1=CC=C(C=C1)[B-](C1=CC=C(Cl)C=C1)(C1=CC=C(Cl)C=C1)C1=CC=C(Cl)C=C1
Solubility Chemicals:
Soluble in chloroform.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) T. Rosatzin, et al.; Anal. Chim. Acta 280, 197 (1993) | (2) K. Suzuki, et al.; Anal. Chem. 67, 324 (1995) | (3) J.S. Benco, et al.; Anal. Chem. 75, 152 (2003)