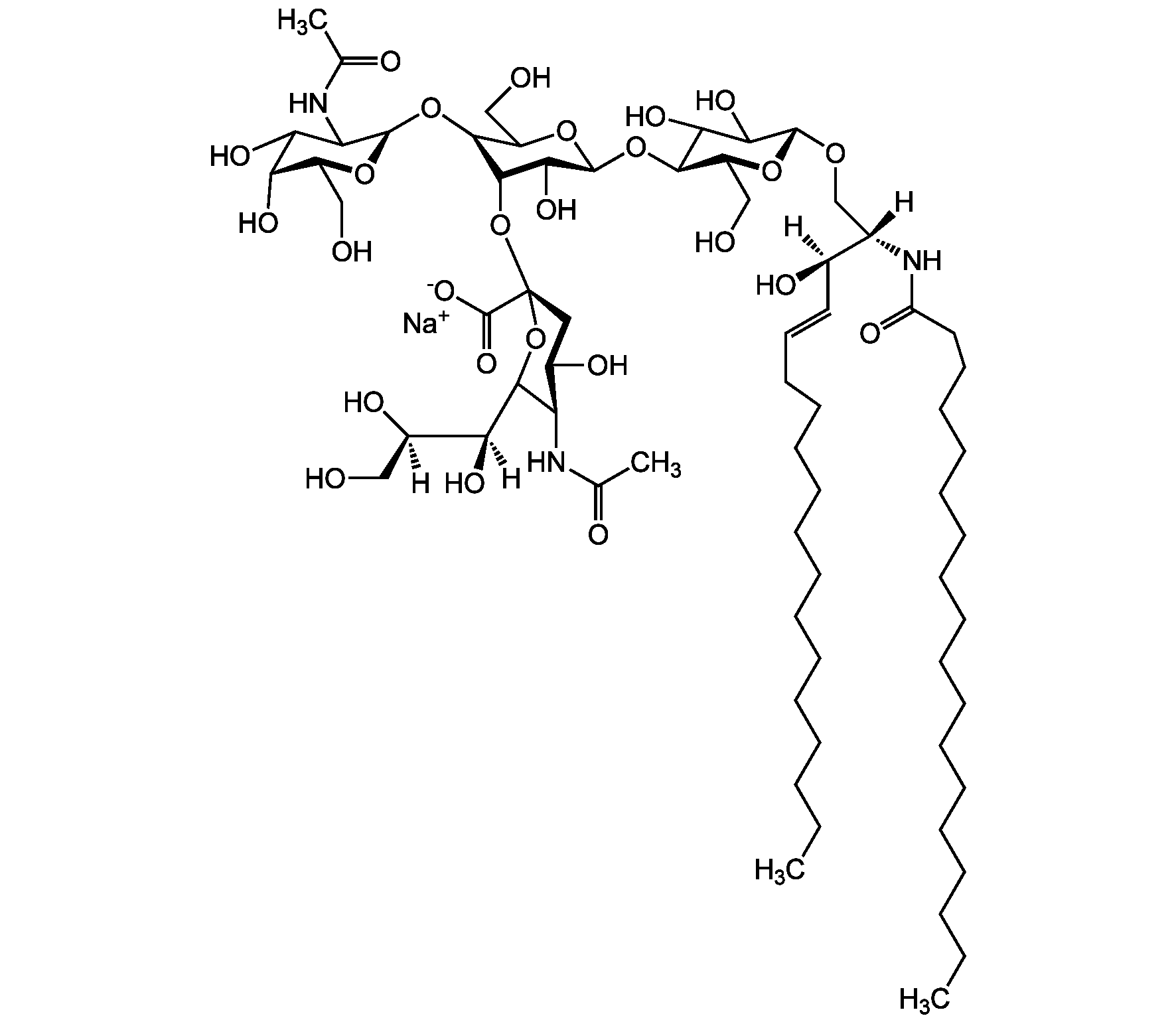
Ganglioside GM2 . sodium salt (bovine brain)
Product Code:
AG-CN2-9001
AG-CN2-9001
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CN2-9001-C500 | 500 ug | £250.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
GM2 . Na; Monosialoganglioside GM2 . Na
CAS:
19600-01-2
EClass:
32160000
Endotoxin:
Not detectable.
Form (Short):
solid
Formulation:
Lyophilized.
Handling Advice:
Hygroscopic.Protect from moisture.
InChi:
InChI=1S/C67H121N3O26.Na/c1-5-7-9-11-13-15-17-19-20-22-24-26-28-30-32-34-50(80)70-43(44(77)33-31-29-27-25-23-21-18-16-14-12-10-8-6-2)40-89-64-57(85)56(84)59(48(38-73)91-64)93-65-58(86)62(60(49(39-74)92-65)94-63-52(69-42(4)76)55(83)54(82)47(37-72)90-63)96-67(66(87)88)35-45(78)51(68-41(3)75)61(95-67)53(81)46(79)36-71;/h31,33,43-49,51-65,71-74,77-79,81-86H,5-30,32,34-40H2,1-4H3,(H,68,75)(H,69,76)(H,70,80)(H,87,88);/q;+1/p-1/b33-31+;/t43-,44+,45?,46+,47?,48?,49?,51+,52?,53-,54-,55?,56?,57?,58?,59+,60-,61?,62?,63+,64+,65-,67-;/m0./s1
InChiKey:
YZCIBJIDUSQSAE-AGTWZOCMSA-M
Long Description:
Chemical. CAS: 19600-01-2. Formula: C67H120N3O26 . Na. MW: 1383.7 . 23.0 (calculated on sphingosine C18:1 and stearic acid). Isolated from bovine brain. Gangliosides are acidic glycosphingolipids that form lipid rafts in the outer leaflet of the cell plasma membrane, especially in neuronal cells in the central nervous system. They participate in cellular proliferation, differentiation, adhesion, signal transduction, cell-to-cell interactions, tumorigenesis and metastasis. The accumulation of gangliosides has been linked to several diseases. Ganglioside GM2 is a very minor component of the nervous system, but it is accumulated in brains from Tay-Sachs and Sandhoff disease patients, due to genetic defect of lysosomal beta-hexosaminidase.
MDL:
MFCD00131137
Molecular Formula:
C67H120N3O26 . Na
Molecular Weight:
1383.7 . 23.0 (calculated on sphingosine C18:1 and stearic acid)
Package Type:
Glass Vial
Product Description:
Gangliosides are acidic glycosphingolipids that form lipid rafts in the outer leaflet of the cell plasma membrane, especially in neuronal cells in the central nervous system. They participate in cellular proliferation, differentiation, adhesion, signal transduction, cell-to-cell interactions, tumorigenesis and metastasis. The accumulation of gangliosides has been linked to several diseases. Ganglioside GM2 is a very minor component of the nervous system, but it is accumulated in brains from Tay-Sachs and Sandhoff disease patients, due to genetic defect of lysosomal beta-hexosaminidase.
Purity:
>98% (TLC)
Sequence:
Structure: II3Neu5AcGgOse3Cer; beta-GalNAc-(1-4)-[alpha-Neu5Ac-(2-3)-]beta-Gal-(1-4)-beta-Glc-(1-1)-Cer; Cer: Sphingosine C18:1-C20:1, ~1:1 to 1:3 by vol.; stearic acid over 90%
SMILES:
[Na+].[H][C@@](O)(CO)[C@]([H])(O)C1O[C@@](CC(O)[C@H]1NC(C)=O)(O[C@@H]1C(O)[C@H](O[C@H]2C(O)C(O)[C@H](OC[C@]([H])(NC(=O)CCCCCCCCCCCCCCCCC)[C@]([H])(O)C=CCCCCCCCCCCCCC)O[C@H]2CO)OC(CO)[C@@H]1O[C@H]1O[C@@H](CO)[C@H](O)C(O)C1NC(C)=O)C([O-])=O
Solubility Chemicals:
Soluble in water (liposomal aggregates) or chloroform:methanol (2:1).
Source / Host:
Isolated from bovine brain.
Transportation:
Non-hazardous
UNSPSC Category:
Glycolipids/Phospholipids/Sphingolipids
UNSPSC Number:
12352211
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Biochemistry and genetics of gangliosidoses: K. Sandhoff & H.Christomanou; Hum. Genet. 50, 107 (1979) | Role of membrane gangliosides in the binding and action of bacterial toxins: P.H. Fishman; J. Membr. Biol. 69, 85 (1982) | Dynamic and structural properties of sphingolipids as driving force to the formation of membrane domains: S. Sonnino, et al.; Chem. Rev. 106, 2111 (2006)