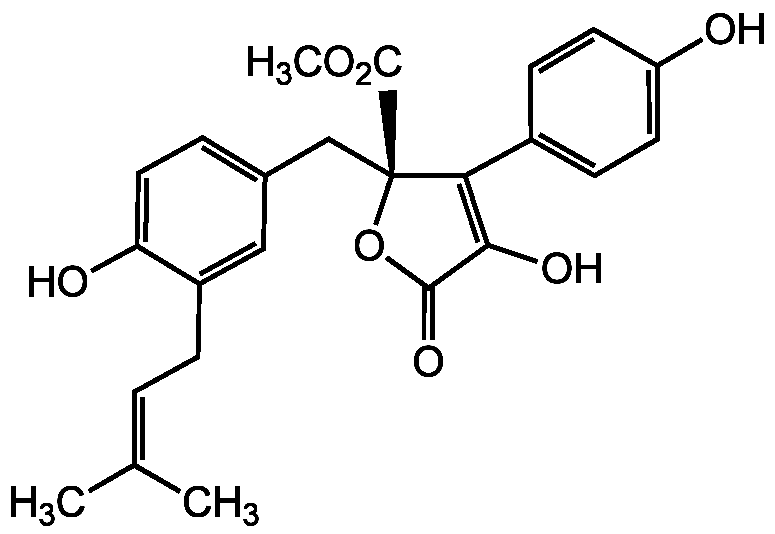
Butyrolactone I
| Code | Size | Price |
|---|
| BVT-0448-C200 | 200 ug | £110.00 |
Quantity:
| BVT-0448-M001 | 1 mg | £150.00 |
Quantity:
| BVT-0448-M005 | 5 mg | £460.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Appearance:
White to off-white solid.
CAS:
87414-49-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light when in solution.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C24H24O7/c1-14(2)4-6-17-12-15(5-11-19(17)26)13-24(23(29)30-3)20(21(27)22(28)31-24)16-7-9-18(25)10-8-16/h4-5,7-12,25-27H,6,13H2,1-3H3
InChiKey:
NGOLMNWQNHWEKU-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 87414-49-1. Formula: C24H24O7. MW: 424.5. Isolated from Aspergillus terreus. Cell permeable, potent and selective cyclin-dependent kinase CDK-1 (CDC2), -2 and -5 inhibitor. Apoptosis inducer. Antitumor agent. Inhibits cell cycle progression at the G1/S and G2/M transitions. Shown to prevent the phosphorylation of retinoblastoma protein and H1 histone. Important probe for understanding the cellular roles of CDKs. Inhibitor of alpha-glucosidases. Maturation inhibitor of sheep oocytes.
MDL:
MFCD03453074
Molecular Formula:
C24H24O7
Molecular Weight:
424.5
Package Type:
Plastic Vial
Precautions:
P261, P271, P280, P312
Product Description:
Cell permeable, potent and selective cyclin-dependent kinase CDK-1 (CDC2), -2 and -5 inhibitor [1-4, 9, 10]. Apoptosis inducer [6, 7, 9]. Antitumor agent [5, 9]. Inhibits cell cycle progression at the G1/S and G2/M transitions [8]. Shown to prevent the phosphorylation of retinoblastoma protein and H1 histone [2, 10]. Important probe for understanding the cellular roles of CDKs [4]. Inhibitor of alpha-glucosidases [12]. Maturation inhibitor of sheep oocytes [11].
Purity:
>98% (HPLC, NMR)
Signal word:
Warning
SMILES:
COC(=O)[C@]1(CC2=CC=C(O)C(CC=C(C)C)=C2)OC(=O)C(O)=C1C1=CC=C(O)C=C1
Solubility Chemicals:
Soluble in DMSO, methanol, ethanol or acetone.
Source / Host:
Isolated from Aspergillus terreus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C. Working aliquots are stable for up to 3 months when stored at -20°C.
References
Butyrolactone I, a selective inhibitor of cdk2 and cdc2 kinase: M. Kitagawa, et al.; Oncogene 8, 2425 (1993) | A cyclin-dependent kinase inhibitor, butyrolactone I, inhibits phosphorylation of RB protein and cell cycle progression: M. Kitagawa, et al.; Oncogene 9, 2549 (1994) | Evidence for cdk5 as a major activity phosphorylating tau protein in porcine brain extract: T. Hosoi, et al.; J. Biochem. 117, 741 (1995) | Chemical inhibitors of cyclin-dependent kinases: L. Meijer; Prog. Cell Cycle Res. 1, 351 (1995) (Review) | Antitumor effects of butyrolactone I, a selective cdc2 kinase inhibitor, on human lung cancer cell lines: K. Nishio, et al.; Anticancer Res. 16, 3387 (1996) | Transcriptional activation of the cdc2 gene is associated with Fas- induced apoptosis of human hematopoietic cells: Y. Furukawa, et al.; J. Biol. Chem. 271, 28469 (1996) | An exogenous cdk inhibitor, butyrolactone-I, induces apoptosis with increased Bax/Bcl-2 ratio in p53-mutated pancreatic cancer cells: M. Wada, et al.; Anticancer Res. 18, 2559 (1998) | Butyrolactone I induces cyclin B1 and causes G2/M arrest and skipping of mitosis in human prostate cell lines: M. Suzuki, et al.; Cancer Lett. 138, 121 (1999) | Involvement of Cdk5/p25 in digoxin-triggered prostate cancer cell apoptosis: H. Lin, et al.; J. Biol. Chem. 279, 29302 (2004) | Regulation of mitotic function of Chk1 through phosphorylation at novel sites by cyclin-dependent kinase 1 (Cdk1): T. Shiromizu, et al.; Genes Cells 11, 477 (2006) | Butyrolactone-I reversibly inhibits but does not improve the maturation and subsequent development of sheep oocytes in vitro: L. Lu, et al.; J. Animal Vet. Adv. 12, 17 (2013) | Effect on alpha-glucosidase inhibition and antioxidant activities of butyrolactone derivatives from Aspergillus terreus MC751: R. Dewi, et al.; Med. Chem. Res. 23, 454 (2014)