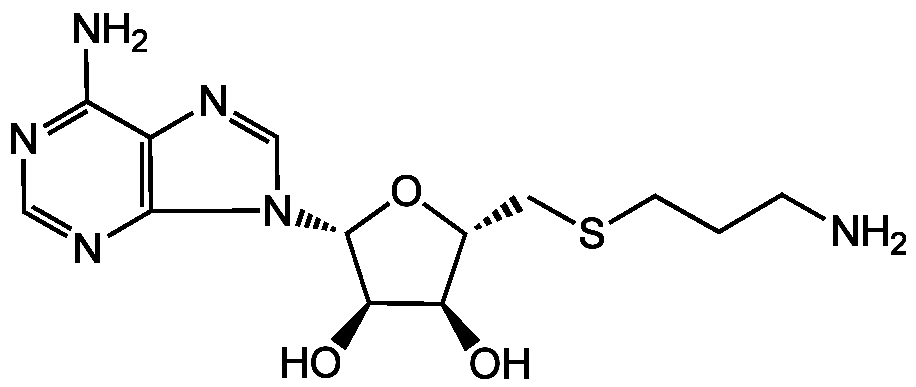
S-(5')-Adenosyl-3-thiopropylamine
| Code | Size | Price |
|---|
| CDX-A0210-M010 | 10 mg | £121.00 |
Quantity:
| CDX-A0210-M050 | 50 mg | £426.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
dc-SAH; dcAdoHcy; Decarboxylated S-Adenosyl-L-homocysteine
Appearance:
White powder.
CAS:
53186-57-5
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H300
InChi:
InChI=1S/C13H20N6O3S/c14-2-1-3-23-4-7-9(20)10(21)13(22-7)19-6-18-8-11(15)16-5-17-12(8)19/h5-7,9-10,13,20-21H,1-4,14H2,(H2,15,16,17)/t7-,9-,10-,13-/m1/s1
InChiKey:
FUSRAALGPJJIRO-QYVSTXNMSA-N
Long Description:
Chemical. CAS: 53186-57-5. Formula: C13H20N6O3S. MW: 340.4. Synthetic This decarboxylated S-Adenosyl-L-methionine is an important metabolite in polyamine biosynthesis, acting as aminopropyl group donor for propylamine transferases such as spermine synthase and spermidine synthase.
MDL:
MFCD17215932
Molecular Formula:
C13H20N6O3S
Molecular Weight:
340.4
Package Type:
Vial
PG:
II
Precautions:
P264, P301, P310
Product Description:
This decarboxylated S-Adenosyl-L-methionine is an important metabolite in polyamine biosynthesis, acting as aminopropyl group donor for propylamine transferases such as spermine synthase and spermidine synthase.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
NCCCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=CN=C2N
Solubility Chemicals:
Soluble in chloroform.
Source / Host:
Synthetic
Transportation:
Excepted Quantity
UN Nummer:
UN 2811
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) S. Ito & J.A. Nicol; Biochem. J. 153, 567 (1976) | (2) K. Samejima & B. Yamanoha; Arch. Biochem. Biophys. 216, 213 (1982) | (3) S. Ito; Meth. Enzymol. 94, 463 (1983) | (4) G. Cacciapuoti, et al.; Eur. J. Biochem. 161, 263 (1986)