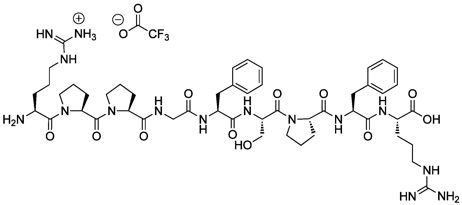
Bradykinin
| Code | Size | Price |
|---|
| AG-CP3-0025-M005 | 5 mg | £42.00 |
Quantity:
| AG-CP3-0025-M025 | 25 mg | £90.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH . TFA
Appearance:
Lyophilized powder.
CAS:
121283-65-6 | 58-82-2 (free base)
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light.
InChi:
InChI=1S/C50H73N15O11.C2HF3O2/c51-32(16-7-21-56-49(52)53)45(72)65-25-11-20-39(65)47(74)64-24-9-18-37(64)43(70)58-28-40(67)59-34(26-30-12-3-1-4-13-30)41(68)62-36(29-66)46(73)63-23-10-19-38(63)44(71)61-35(27-31-14-5-2-6-15-31)42(69)60-33(48(75)76)17-8-22-57-50(54)55;3-2(4,5)1(6)7/h1-6,12-15,32-39,66H,7-11,16-29,51H2,(H,58,70)(H,59,67)(H,60,69)(H,61,71)(H,62,68)(H,75,76)(H4,52,53,56)(H4,54,55,57);(H,6,7)/t32-,33-,34-,35-,36-,37-,38-,39-;/m0./s1
InChiKey:
WTRZIOCXFWDHEM-MIEKKPBHSA-N
Long Description:
Chemical. CAS: 121283-65-6 | 58-82-2 (free base). Formula: C50H73N15O11 . C2HF3O2. MW: 1060.2 . 114.0. Pro-inflammatory peptide, acting through G-protein-coupled receptors. Endogenous bradykinin receptor agonist that displays selectivity for B2 over B1 receptors. Endogenously formed following pathophysiological stimuli such as inflammation, tissue damage or anoxia. Activator of endothelial nitric oxide synthase (eNOS; NOS III). Reduces blood pressure by inducing the release of prostacyclin, nitric oxide and endothelium-derived hyperpolarizing factor. Plays an important role in the regulation of fluid and electrolyte balance, non-vascular smooth muscle contraction, vasodilation and capillary permeability.
MDL:
MFCD00076258
Molecular Formula:
C50H73N15O11 . C2HF3O2
Molecular Weight:
1060.2 . 114.0
Package Type:
Vial
Product Description:
Pro-inflammatory peptide, acting through G-protein-coupled receptors. Endogenous bradykinin receptor agonist that displays selectivity for B2 over B1 receptors. Endogenously formed following pathophysiological stimuli such as inflammation, tissue damage or anoxia. Activator of endothelial nitric oxide synthase (eNOS; NOS III). Reduces blood pressure by inducing the release of prostacyclin, nitric oxide and endothelium-derived hyperpolarizing factor. Plays an important role in the regulation of fluid and electrolyte balance, non-vascular smooth muscle contraction, vasodilation and capillary permeability.
Product Line Areas NEW:
Biochemicals, Immunology, Inflammation, Metabolism
Product Type:
Chemical
Purity:
>97% (HPLC)
Sequence:
H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH
SMILES:
[O-]C(=O)C(F)(F)F.N[C@@H](CCCNC([NH3+])=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O
Solubility Chemicals:
Soluble in water (5mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
Actions of pure bradykinin: D.F. Elliott, et al.; J. Physiol. 153, 473 (1960) | The discovery of nitric oxide as the endogenous nitrovasodilator: S. Moncada, et al.; Hypertension 12, 365 (1988) | Bradykinin and ATP stimulate L-arginine uptake and nitric oxide release in vascular endothelial cells: R.G. Bogle, et al.; BBRC 180, 926 (1991) | Biosynthesis of endothelium-derived nitric oxide by bradykinin as endogenous precursor: A.R. Volpe, et al.; Immunopharmacology 33, 287 (1996) | Bradykinin stimulates NF-kappaB activation and interleukin 1beta gene expression in cultured human fibroblasts: Z.K. Pan, et al.; J. Clin. Invest. 98, 2042 (1996) | Bradykinin and nitric oxide in infectious disease and cancer: H. Maeda, et al.; Immunopharmacol. 33, 222 (1996) | Bradykinin receptors and their antagonists: D. Regoli, et al.; Eur. J. Pharmacol. 348, 1 (1998) | Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation: M.B. Harris, et al.; J. Biol. Chem. 276, 16587 (2001) | International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences: L.M. Leeb-Lundberg, et al.; Pharmacol. Rev. 57, 27 (2005) | Formation of bradykinin: a major contributor to the innate inflammatory response: K. Joseph & A.P. Kaplan; Adv. Immunol. 86, 159 (2005) | Peptide and non-peptide bradykinin receptor antagonists: role in allergic airway disease: W.M. Abraham, et al.; Eur. J. Pharmacol. 533, 215 (2006) | Bradykinin mediates phosphorylation of eNOS in odontoblasts: Y. Korkmaz, et al.; J. Dent. Res. 85, 536 (2006) | Multifunctional effects of bradykinin on glial cells in relation to potential anti-inflammatory effects: M. Noda, et al.; Neurochem. Int. 51, 185 (2007) | Pathogenic mechanisms of bradykinin mediated diseases: Dysregulation of an innate inflammatory pathway: A.P. Kaplan & K. Joseph; Adv. Immunol. 121, 41 (2014)