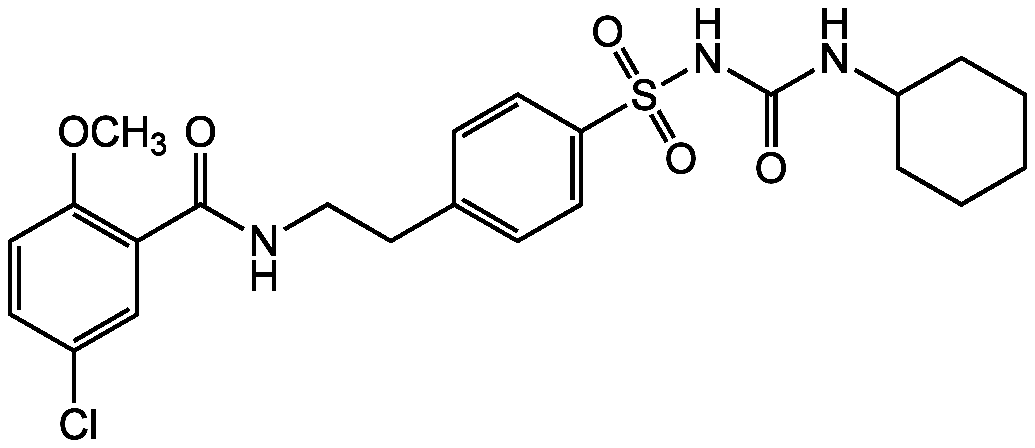
Glyburide
| Code | Size | Price |
|---|
| AG-CR1-3613-G001 | 1 g | £35.00 |
Quantity:
| AG-CR1-3613-G005 | 5 g | £52.00 |
Quantity:
| AG-CR1-3613-G010 | 10 g | £80.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
+4°C
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Diabeta; Glibenclamide
Appearance:
White to off-white solid.
CAS:
10238-21-8
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29)
InChiKey:
ZNNLBTZKUZBEKO-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 10238-21-8. Formula: C23H28ClN3O5S. MW: 494. Antidiabetic compound. Binds to and activates the ATP-sensitive potassium channels (KATP) inhibitory regulatory subunit sulfonylurea receptor 1 (SUR1). Causes cell membrane depolarization and opening of voltage-dependent calcium channel. Increases intracellular calcium and stimulates insulin secretion in beta cells NLRP3 inflammasome inhibitor. Broad-spectrum ATP-binding cassette (ABC) transporter inhibitor. Shown to have anti-leishmanial activity. Potential inhibitor of collagenases.
MDL:
MFCD00056625
Molecular Formula:
C23H28ClN3O5S
Molecular Weight:
494
Package Type:
Vial
Product Description:
Antidiabetic compound. Binds to and activates the ATP-sensitive potassium channels (KATP) inhibitory regulatory subunit sulfonylurea receptor 1 (SUR1). Causes cell membrane depolarization and opening of voltage-dependent calcium channel. Increases intracellular calcium and stimulates insulin secretion in beta cells NLRP3 inflammasome inhibitor. Broad-spectrum ATP-binding cassette (ABC) transporter inhibitor. Shown to have anti-leishmanial activity. Potential inhibitor of collagenases.
Purity:
>98%
SMILES:
COC1=C(C=C(Cl)C=C1)C(=O)NCCC1=CC=C(C=C1)S(=O)(=O)NC(=O)NC1CCCCC1
Solubility Chemicals:
Soluble in DMSO. Slightly soluble (<1mg/ml) in ethanol (gentle warming).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Glyburide: a second-generation sulfonylurea hypoglycemic agent. History, chemistry, metabolism, pharmacokinetics, clinical use and adverse effects: J.M. Feldman; Pharmacotherapy 5, 43 (1985) | The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells: H. Schmid-Antomarchi, et al.; J. Biol. Chem. 262, 15840 (1987) | Antidiabetic sulfonylureas control action potential properties in heart cells via high affinity receptors that are linked to ATP-dependent K+ channels: M. Fosset, et al.; J. Biol. Chem. 263, 7933 (1988) | Sensitivity of a renal K+ channel (ROMK2) to the inhibitory sulfonylurea compound glibenclamide is enhanced by coexpression with the ATP-binding cassette transporter cystic fibrosis transmembrane regulator: C.M. McNicholas, et al.; PNAS 93, 8083 (1996) | The sulphonylurea glibenclamide inhibits multidrug resistance protein (MRP1) activity in human lung cancer cells: L. Payen, et al.; Br. J. Pharmacol. 132, 778 (2001) | Glibenclamide, a blocker of K+(ATP) channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis: X. Serrano-Martin, et al.; Antimicrob. Agents Chemother. 50, 4214 (2006) | CFTR inhibition by glibenclamide requires a positive charge in cytoplasmic loop three: P. Melin, et al.; Biochim. Biophys. Acta 1768, 2438 (2007) | Glyburide inhibits the Cryopyrin/Nalp3 inflammasome: M. Lamkanfi, et al.; J. Cell. Biol. 187, 61 (2009) | Glyburide in treating malignant cerebral edema. Blocking sulfonyl urea one (SUR1) receptors: T.V. Pallan & I. Ahmed; J. Vasc. Interv. Neurol. 7, 23 (2014) | In vitro biological evaluation of glyburide as potential inhibitor of collagenases: V.L. Bodiga, et al.; Int. J. Biol. Macromol. 70, 187 (2014) | NLRP3 inflammasome activation is essential for paraquat-induced acute lung injury: Z. Liu, et al.; Inflammation 38, 433 (2015)
Related Products
| Product Name | Product Code | Supplier | Colchicine | AG-CN2-0048 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parthenolide | AG-CN2-0455 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arglabin | AG-CN2-0458 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BAY 11-7082 | AG-CR1-0013 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||