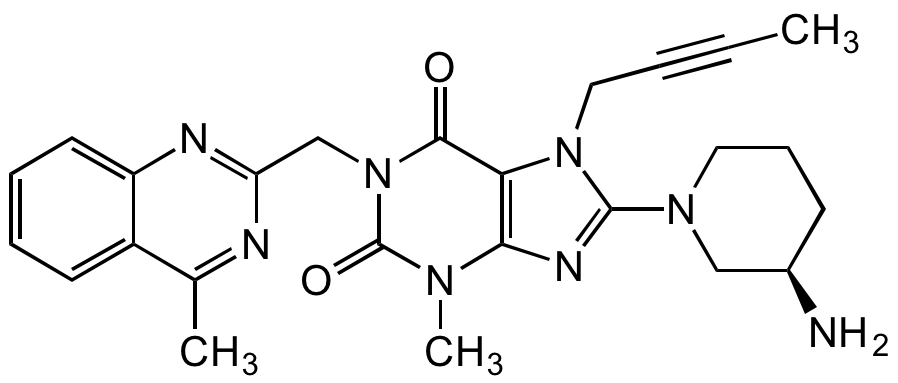
Linagliptin
| Code | Size | Price |
|---|
| AG-CR1-3618-M010 | 10 mg | £50.00 |
Quantity:
| AG-CR1-3618-M050 | 50 mg | £90.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
8-(3-(R)-Aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydropurine-2,6-dione; BI-1356
Appearance:
White to off-white solid.
CAS:
668270-12-0
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1
InChiKey:
LTXREWYXXSTFRX-QGZVFWFLSA-N
Long Description:
Chemical. CAS: 668270-12-0. Formula: C25H28N8O2. MW: 472.5. Antidiabetic agent. Highly potent and selective competitive inhibitor of dipeptidyl-peptidase 4 (DPP4; DPP IV; CD26), an enzyme that degrades, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Prevents the inactivation of endogenous GLP-1 and GIP. Shown to restore beta cell function and survival in human isolated islets through GLP-1 stabilization. Improves insulin sensitivity. Anti-inflammatory compound.
MDL:
MFCD14635356
Molecular Formula:
C25H28N8O2
Molecular Weight:
472.5
Package Type:
Vial
Precautions:
P261, P271, P280, P312
Product Description:
Antidiabetic agent. Highly potent and selective competitive inhibitor of dipeptidyl-peptidase 4 (DPP4; DPP IV; CD26), an enzyme that degrades, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Prevents the inactivation of endogenous GLP-1 and GIP. Shown to restore beta cell function and survival in human isolated islets through GLP-1 stabilization. Improves insulin sensitivity. Anti-inflammatory compound. DPP4 is a cell surface aminopeptidase that exerts diverse biological activities, such as protease activity, association with adenosine deaminase, interaction with the extracellular matrix, regulation of intracellular signal transduction coupled with the control of cell migration and proliferation, and in addition cell surface co-receptor activity mediating viral entry. DPP4 is a viral receptor of human coronaviruses and therefore is investigated as a potential target for SARS-CoV-2 infections.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
CC#CCN1C(=NC2=C1C(=O)N(CC1=NC3=CC=CC=C3C(C)=N1)C(=O)N2C)N1CCC[C@@H](N)C1
Solubility Chemicals:
Soluble in DMSO (5mg/ml), ethanol or methanol. Slightly soluble in water (~1mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
8-(3-(R)-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a highly potent, selective, long-acting, and orally bioavailable DPP-4 inhibitor for the treatment of type 2 diabetes: M. Eckhardt, et al.; J. Med. Chem. 50, 6450 (2007) | Safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of BI 1356, an inhibitor of dipeptidyl peptidase 4, in healthy male volunteers: S. H?ttner, et al.; J. Clin. Pharmacol. 48, 1171 (2008) | (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors: L. Thomas, et al.; J. Pharmacol. Exp. Ther. 325, 175 (2008) | Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients: T. Heise, et al.; Diabetes Obes. Metab. 11, 786 (2009) | The DPP4 inhibitor linagliptin delays the onset of diabetes and preserves beta-cell mass in non-obese diabetic mice: J. Jelsing, et al.; J. Endocrinol. 214, 381 (2012) | The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice: C. Schurmann, et al.; J. Pharmacol. Exp. Ther. 342, 71 (2012) | Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity: M. Kern, et al.; PLoS One 7, e38744 (2012) | Emerging DPP-4 inhibitors: focus on linagliptin for type 2 diabetes: B. Gallwitz; Diabetes Metab. Syndr. Obes. 6, 1 (2013) (Review) | The DPP-4 inhibitor linagliptin restores beta-cell function and survival in human isolated islets through GLP-1 stabilization: P. Shah, et al.; J. Clin. Endocrinol. Metab. 98, E1163 (2013) | Linagliptin: from bench to bedside: J. Doupis; Drug. Des. Devel. Ther. 8, 431 (2014) (Review) | Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses: F. Qi, et al.; BBRC (Epub ahead of print) (2020)