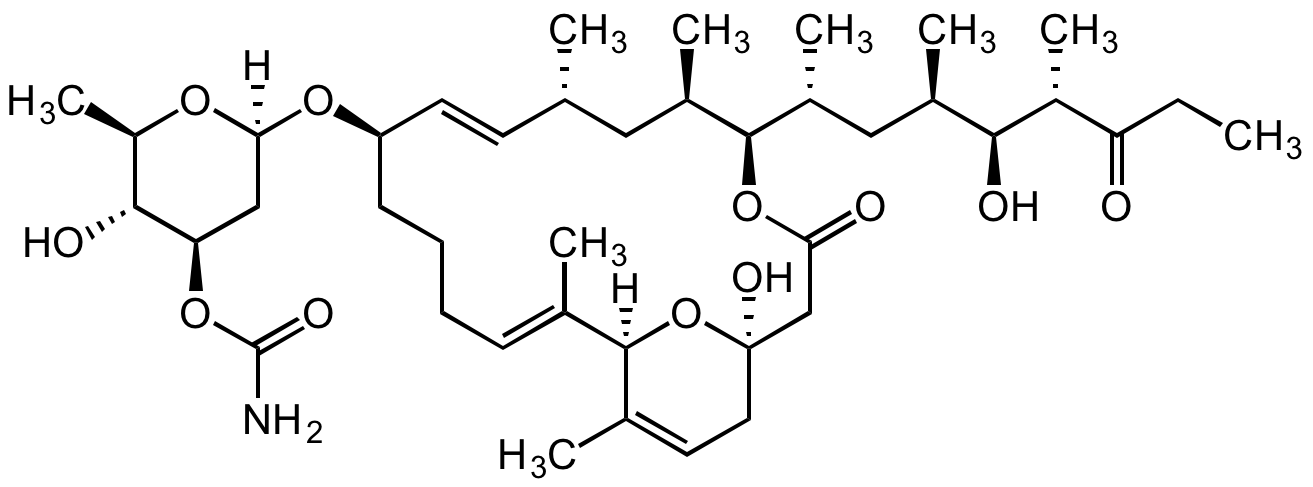
Venturicidin A
| Code | Size | Price |
|---|
| BVT-0454-M001 | 1 mg | £140.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Aabomycin A1
Appearance:
White solid.
CAS:
33538-71-5
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light when in solution.
Hazards:
H302
InChi:
InChI=1S/C41H67NO11/c1-10-32(43)29(8)36(45)26(5)20-28(7)38-27(6)19-23(2)15-16-31(50-35-21-33(51-40(42)47)37(46)30(9)49-35)14-12-11-13-24(3)39-25(4)17-18-41(48,53-39)22-34(44)52-38/h13,15-17,23,26-31,33,35-39,45-46,48H,10-12,14,18-22H2,1-9H3,(H2,42,47)/b16-15+,24-13+/t23-,26+,27+,28+,29+,30+,31+,33+,35-,36-,37+,38-,39+,41+/m0/s1
InChiKey:
HHQKNFDAEDTRJK-LIOWZGMGSA-N
Long Description:
Chemical. CAS: 33538-71-5. Formula: C41H67NO11. MW: 750. Isolated from Streptomyces aureofaciens DSM 40731. 20-Membered macrolide glycoside. Antibiotic and antifungal agent. Shows antitrypanosomal and antimalarial activity. Potent inhibitor of mitochondrial and bacterial ATP synthase. Inhibitor of F0F1-ATPase. Na+-translocating ATP synthases inhibitor.
MDL:
MFCD00036323
Molecular Formula:
C41H67NO11
Molecular Weight:
750
Package Type:
Plastic Vial
Precautions:
P264, P270, P301, P312
Product Description:
20-Membered macrolide glycoside. Antibiotic and antifungal agent. Shows antitrypanosomal and antimalarial activity. Potent inhibitor of mitochondrial and bacterial ATP synthase. Inhibitor of F1F0-ATPase. Na+-translocating ATP synthases inhibitor.
Purity:
>98% (1H-NMR, HPLC)
Signal Word:
Warning
SMILES:
[H][C@@]1(C[C@@H](OC(N)=O)[C@H](O)[C@@H](C)O1)O[C@@H]1CCCC=C(C)[C@@]2([H])O[C@](O)(CC=C2C)CC(=O)O[C@H]([C@H](C)C[C@@H](C)[C@H](O)[C@H](C)C(=O)CC)[C@H](C)C[C@@H](C)C=C1
Solubility Chemicals:
Soluble in DMSO, methanol, acetone or dichloromethane. Insoluble in hexane or water.
Source / Host:
Isolated from Streptomyces aureofaciens.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C.
References
Venturicidin: a new antifungal antibiotic of potential use in agriculture: A. Rhodes, et al.; Nature 192, 952 (1961) | Metabolic products of microorganismus. 102. The structure of venturicidin A and B: M. Brufani, et al.; Helv. Chim. Acta 55, 2329 (1972) | The chemotherapy of rodent malaria, XXXI. The effect of some metabolic inhibitors upen chloroquine-induced pigment clumping (CIPC) in Plasmodium berghei: D. C. Warhurst, et al.; Ann. Trop. Med. Parasitol. 72, 203 (1978) | Effects of inhibitors on mitochondrial adenosine triphosphatase of T. pyriformis ST.: M. D. Unitt, et al.; J. Gen. Microbiol. 126, 261 (1981) | Inhibition of Escherichia coli H+-ATPase by venturicidin, oligomycin and ossamycin: D. S. Perlin, et al.; Biochim. Biophys. Acta 807, 238 (1985) | Identity of aabomycin A with venturicidins: H. Akita, et al.; Agric. Biol. Chem. 54, 2465 (1990) | An attempt to discriminate catalytic and regulatory proton binding sites in membrane-bound, thiol-reduced chloroplast ATP: M. Valerio, et al.; Biochemistry 31, 4239 (1992) | Studies on the mechanism of oxidative phosphorylation. ATP synthesis by submitochondrial particles inhibited at F0 by venturicidin and organotin compounds: A. Matsuno-Yagi & Y. Hatefi; J. Biol. Chem. 268, 6168 (1993) | The prokaryotic thermophilic TF1-ATPase is functionally compatible with the eukaryotic CF0-part of the chloroplast ATP sythase: J. M. Galmiche, et al.; FEBS Lett. 338, 152 (1994) | Modification of sulfhydryl groups in the g-subunit of chloroplast-coupling factor 1 affects the proton slip through the ATP synthase: Y. Evron, et al.; Plant Physiol. 115, 1549 (1997) | Selective and potent in vitro antitrypanosomal activities of ten microbial metabolites: K. Otoguro, et al.; J. Antibiot. 61, 372 (2008) | A novel 11-kDa inhibitory subunit in the F1FO ATP synthase of Paracoccus denitrificans and related alpha-proteobacteria: E. Morales-Rios, et al.; FASEB J. 24, 599 (2010) | Venturicidin C, a new 20-membered macrolide produced by Streptomyces sp. TS-2-2: K. Shaaban et al.; J. Antibiot. 67, 223 (2014)