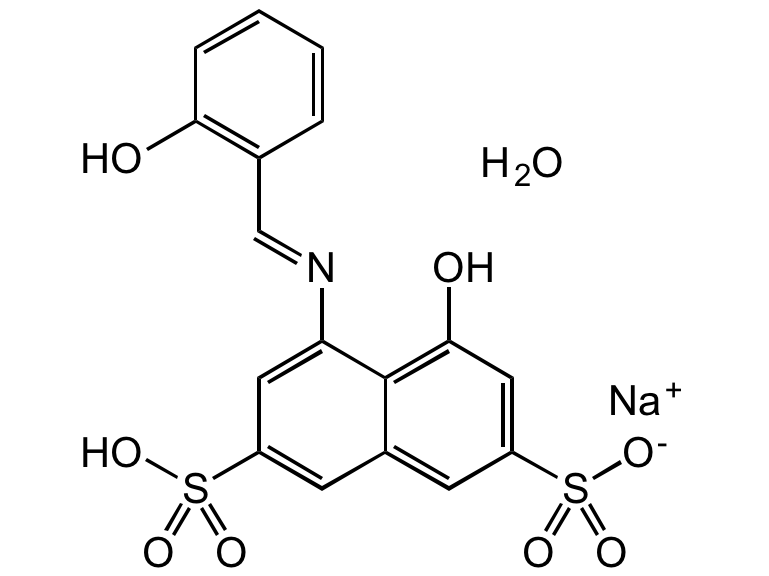
Azomethine-H monosodium salt hydrate
| Code | Size | Price |
|---|
| CDX-A0149-G005 | 5 g | £108.00 |
Quantity:
| CDX-A0149-G025 | 25 g | £401.00 |
Quantity:
| CDX-A0149-G100 | 100 g | £1,109.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
4-Hydroxy-5-(2-hydroxybenzylideneamino)-naphthalene-2,7-disulfonic acid monosodium salt hydrate; 4-Hydroxy-5-(salicylideneamino)-2,7-naphthalenedisulfonic acid monosodium salt
Appearance:
Yellow to orange powder.
CAS:
206752-32-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C17H13NO8S2.Na.H2O/c19-15-4-2-1-3-10(15)9-18-14-7-12(27(21,22)23)5-11-6-13(28(24,25)26)8-16(20)17(11)14;;/h1-9,19-20H,(H,21,22,23)(H,24,25,26);;1H2/q;+1;/p-1/b18-9+;;
InChiKey:
AAIGDXDVSZJVSW-IFJQNBRBSA-M
Long Description:
Chemical. CAS: 206752-32-1. Formula: C17H12NNaO8S2 . xH2O. MW: 445.40 (anhydrous basis). Synthetic Probe for the colorimetric determination of boron in samples such us soils, plants, composts, manure, water, nutirent solution, glass or steel (microgram levels of boron). It forms an orange complex with boron in aqueous solution (absorption maxima at ~415nm). The detection range of boron in sample solutions is 1.0-6ppm. To detect boron in plant samples EDTA is used to mask copper, iron and aluminium ions. It is also used in electrocyclization reactions in the synthesis of martinellic acid, spirotryprostatin A and benzodiazepinones. Was shown to produce free radicals and might have anti-malarial and anti-cancer properties.
MDL:
MFCD00149588
Molecular Formula:
C17H12NNaO8S2 . xH2O
Molecular Weight:
445.40 (anhydrous basis)
Package Type:
Vial
Precautions:
P261, P305, P351, P338
Product Description:
Probe for the colorimetric determination of boron in samples such us soils, plants, composts, manure, water, nutirent solution, glass or steel (microgram levels of boron). It forms an orange complex with boron in aqueous solution (absorption maxima at ~415nm). The detection range of boron in sample solutions is 1.0-6ppm. To detect boron in plant samples EDTA is used to mask copper, iron and aluminium ions. It is also used in electrocyclization reactions in the synthesis of martinellic acid, spirotryprostatin A and benzodiazepinones. Was shown to produce free radicals and might have anti-malarial and anti-cancer properties.
Purity:
>95% (T)
Signal word:
Warning
SMILES:
O.[Na+].OC1=C(C=NC2=CC(=CC3=CC(=CC(O)=C23)S([O-])(=O)=O)S(O)(=O)=O)C=CC=C1
Solubility Chemicals:
Soluble in water, ethanol or acetone.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) W.D. Basson, et al.; Analyst 94, 1135 (1969) | (2) R.R. Spencer & D.E. Erdmann; Environ. Sci. Technol. 13, 954 (1979) | (3) R.A. Edwards; Analyst 105, 139 (1980) | (4) M. Zenki, et al.; Fresenius J. Anal. Chem. 334, 238 (1989) | (5) J. Ciba & A. Chrusciel; Fresenius J. Anal. Chem. 342, 147 (1992) | (6) E.L. Novelli, et al.; Free Radic. Res. 26, 319 (1997) | (7) A. Gross, et al.; Chemosphere 72, 400 (2008) | (8) E. Merdivan, et al.; Spectrochim. Acta A Mol. Biomol. Spectrosc. 71, 2045 (2009)