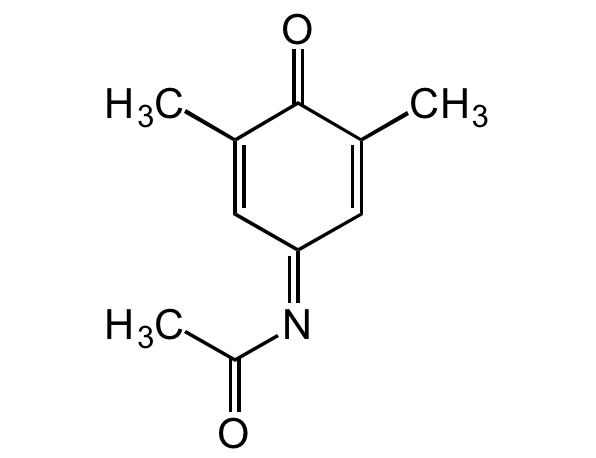
3,5-Dimethyl-NAPQI
| Code | Size | Price |
|---|
| CDX-A0163-M010 | 10 mg | £242.00 |
Quantity:
| CDX-A0163-M025 | 25 mg | £474.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NA-3,5-Bqi; N-Acetyl-3,5-dimethyl-4-benzoquinone imine
Appearance:
Orange powder.
CAS:
74827-85-3
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C10H11NO2/c1-6-4-9(11-8(3)12)5-7(2)10(6)13/h4-5H,1-3H3
InChiKey:
VUPORYDINWWWKZ-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 74827-85-3. Formula: C10H11NO2. MW: 177.2. Synthetic Metabolite. Oxidizing analog of N-acetyl-p-benzoquinone imine (NAPQI), a toxic metabolite of acetaminophen (paracetamol). Primarily oxidizes protein thiols. Shows cytotoxic properties, through disruption of intracellular Ca2+ homeostasis. Compound can be used as analytical reference material.
MDL:
MFCD00078877
Molecular Formula:
C10H11NO2
Molecular Weight:
177.2
Package Type:
Vial
Precautions:
P261, P305, P351, P338
Product Description:
Metabolite. Oxidizing analog of N-acetyl-p-benzoquinone imine (NAPQI), a toxic metabolite of acetaminophen (paracetamol). Primarily oxidizes protein thiols. Shows cytotoxic properties, through disruption of intracellular Ca2+ homeostasis. Compound can be used as analytical reference material.
Purity:
>95.0% (HPLC)
Signal word:
Warning
SMILES:
CC(=O)N=C1C=C(C)C(=O)C(C)=C1
Solubility Chemicals:
Soluble in chloroform.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) R. van de Straat, et al.; Biochem. Pharmacol. 36, 2065 (1987) | (2) L. Rossi, et al.; Mol. Pharmacol. 34, 674 (1988) | (3) P. Nicotera, et al.; Chem. Res. Toxicol. 2, 46 (1989) | (4) P. Nicotera, et al.; Arch. Biochem. Biophys. 283, 200 (1990)