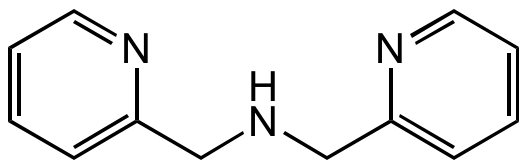
Di-(2-picolyl)amine
| Code | Size | Price |
|---|
| CDX-B0134-GG25 | 2.5 g | £59.00 |
Quantity:
| CDX-B0134-G005 | 5 g | £96.00 |
Quantity:
| CDX-B0134-G025 | 25 g | £353.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Dipicolylamine; DPA; Bis(2-pyridylmethyl)amine; 2,2'-Bis(pyridylmethyl)amine; NSC 176070
Appearance:
Yellow to orange liquid.
CAS:
1539-42-0
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C12H13N3/c1-3-7-14-11(5-1)9-13-10-12-6-2-4-8-15-12/h1-8,13H,9-10H2
InChiKey:
KXZQYLBVMZGIKC-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1539-42-0. Formula: C12H13N3. MW: 199.25. Synthetic Building block for synthesis. DPA is a secondary amine with two picolyl substituents. The compound is a tridentate ligand in coordination chemistry and commonly used to produce Zn-based chemosensors/probes, such as Zinpry. As a tridentate ligand this compound provides three nitrogen donors that affords good selectivity for Zn2+ over biologically relevant metals such as Na+, K+, Mg2+ and Ca2+, and leaves coordination sites free for anion binding. The amino nitrogen of the DPA group is a good candidate as an electron donor in either photoinduced electron transfer or photoinduced charge transfer (PET or PCT) sensors. Zn(II)?DPA complexes are widely used in anion recognition and sensing.
MDL:
MFCD00129044
Molecular Formula:
C12H13N3
Molecular Weight:
199.25
Package Type:
Vial
Precautions:
P261, P305, P351, P338
Product Description:
Building block for synthesis. DPA is a secondary amine with two picolyl substituents. The compound is a tridentate ligand in coordination chemistry and commonly used to produce Zn-based chemosensors/probes, such as Zinpry. As a tridentate ligand this compound provides three nitrogen donors that affords good selectivity for Zn2+ over biologically relevant metals such as Na+, K+, Mg2+ and Ca2+, and leaves coordination sites free for anion binding. The amino nitrogen of the DPA group is a good candidate as an electron donor in either photoinduced electron transfer or photoinduced charge transfer (PET or PCT) sensors. Zn(II)?DPA complexes are widely used in anion recognition and sensing.
Purity:
>95% (GC)
Signal word:
Warning
SMILES:
C(NCC1=CC=CC=N1)C1=CC=CC=N1
Solubility Chemicals:
Sligthly soluble in water.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) E.M. Nolan & S.J. Lippard; Inorg. Chem. 43, 8310 (2004) | (2) N.C. Lim & C. Bruckner; Chem. Commun. 2004, 1094 (2004) | (3) H.-W. Lee, et al.; Bull. Korean Chem. Soc. 28, 855 (2007) | (4) L. Xue, et al.; Inorg. Chem. 47, 4310 (2008) | (5) T. Sakamoto, et al.; Chem. Commun. 2009, 141 (2009) | (6) L. You, et al.; Nat. Chem. 3, 943 (2011) | (7) H.T. Ngo, et al.; Chem. Soc. Rev. 41, 4928 (2012)
Related Products
| Product Name | Product Code | Supplier | (1S)-(+)-3-Bromocamphor-10-sulfonic acid hydrate | CDX-B0135 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bis(4-methylphenyl)iodonium hexafluorophosphate | CDX-B0247 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5-Bromopyridine-3-carboxylic acid | CDX-B0248 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3-Bromo-1-butyne | CDX-B0250 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||