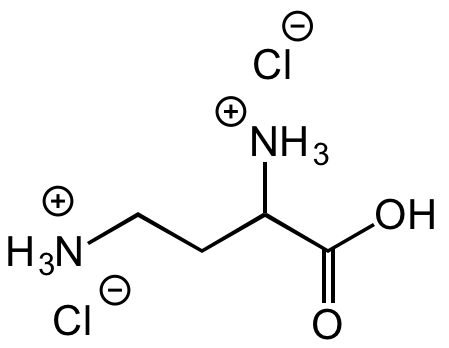
L-2,4-Diaminobutyric acid dihydrochloride
| Code | Size | Price |
|---|
| CDX-D0154-G001 | 1 g | £65.00 |
Quantity:
| CDX-D0154-G005 | 5 g | £205.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(2S)-2,4-Diamino-butanoic acid dihydrochloride; H-Dab-OH
Appearance:
Yellowish powder.
CAS:
1883-09-6
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS05,GHS07
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H315, H318, H335
InChi:
InChI=1S/C4H10N2O2.2ClH/c5-2-1-3(6)4(7)8;;/h3H,1-2,5-6H2,(H,7,8);2*1H
InChiKey:
CKAAWCHIBBNLOJ-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1883-09-6. Formula: C4H10N2O2 . 2HCl. MW: 118.1 . 72.9. Synthetic Unnatural amino acid derivative. Pharmacological tool and potential chiral building block used as an internal standard for amino acid analysis. Suitable reagent used for the differentiation of beta-N-methylamino-L-alanine from the diamino acids by using HPLC-FD, UHPLC-UV, UHPLC-MS, and triple quadrupole tandem mass spectrometry (UHPLC-MS/MS). Used in the quantification of neurotoxin beta-N-methylamino-L-alanine (BMAA) in seafood. It has been found to inhibit GABA transaminase (ABAT), producing elevated levels of GABA and to have an antitumor activity.
MDL:
MFCD00064561
Molecular Formula:
C4H10N2O2 . 2HCl
Molecular Weight:
118.1 . 72.9
Package Type:
Vial
Precautions:
P261, P280, P305, P351, P338
Product Description:
Unnatural amino acid derivative. Pharmacological tool and potential chiral building block used as an internal standard for amino acid analysis. Suitable reagent used for the differentiation of beta-N-methylamino-L-alanine from the diamino acids by using HPLC-FD, UHPLC-UV, UHPLC-MS, and triple quadrupole tandem mass spectrometry (UHPLC-MS/MS). Used in the quantification of neurotoxin beta-N-methylamino-L-alanine (BMAA) in seafood. It has been found to inhibit GABA transaminase (ABAT), producing elevated levels of GABA and to have an antitumor activity.
Purity:
>95% (NMR)
Signal word:
Danger
SMILES:
[Cl-].[Cl-].[NH3+]CCC([NH3+])C(O)=O
Solubility Chemicals:
Soluble in water.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Chiral Reagents
UNSPSC Number:
12352100
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) J.F. Riordan & R.W. Giese; Meth. Enzymol. 47, 31 (1977) | (2) P.M. Beart, et al.; Neurosci. Lett. 5, 193 (1977) | (3) G. Ronquist, et al.; J. Cancer Res. Clin. Oncol. 96, 259 (1980) | (4) P.J. Blind, et al.; Anticancer Res. 23, 1245 (2003) | (5) M.G. Thomas; J. Bacteriol. 191, 4594 (2009) | (6) S.A. Banack, et al.; Toxicon. 57, 730 (2011) | (7) L. Jiang, et al.; Sci. Rep. 4, 6931 (2014)
Related Products
| Product Name | Product Code | Supplier | 1(E)-Iodo-oct-1-ene | CDX-I0037 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1(E)-Iodo-hex-1-ene | CDX-I0047 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D-(+)-Melezitose monohydrate | CDX-M0021 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2-Maleinimidoethyl mesylate | CDX-M0078 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| N-Phenylhydroxylamine | CDX-P0103 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||