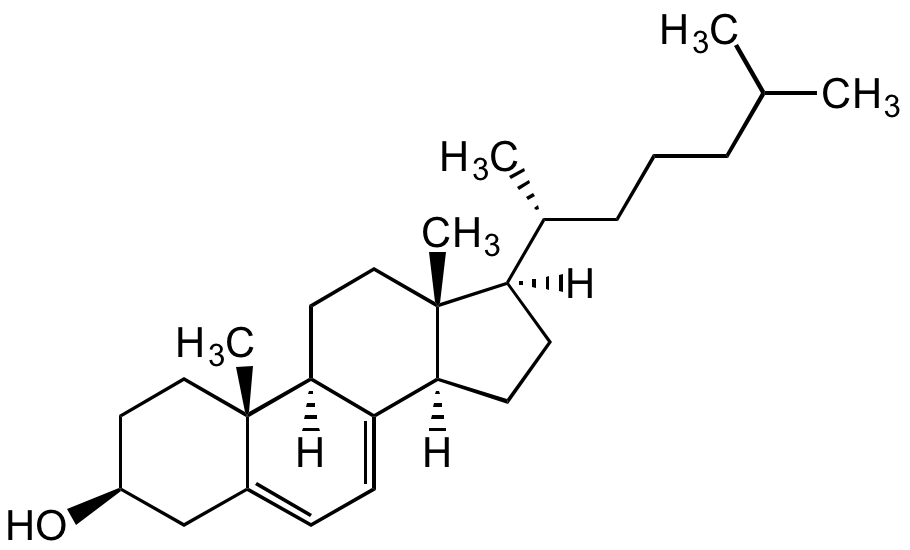
7-Dehydrocholesterol
| Code | Size | Price |
|---|
| CDX-D0331-G010 | 10 g | £89.00 |
Quantity:
| CDX-D0331-G025 | 25 g | £169.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
7-DHC; NSC 18159; Provitamin D3; Delta7-Cholesterol; (-)-7-Dehydrocholesterol; 3beta-Hydroxy-5,7-cholestadiene; 5,7-Cholestadien-3beta-ol
Appearance:
White powder.
CAS:
434-16-2
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C27H44O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9-10,18-19,21,23-25,28H,6-8,11-17H2,1-5H3/t19-,21+,23-,24+,25+,26+,27-/m1/s1
InChiKey:
UCTLRSWJYQTBFZ-DDPQNLDTSA-N
Long Description:
Chemical. CAS: 434-16-2. Formula: C27H44O. MW: 384.64. Synthetic Precursor of cholesterol and vitamin D3. It is reduced to cholesterol by the enzyme 3beta-hydroxysterol-Delta7-reductase (DHCR7) in the last step of cholesterol biosynthesis. Mutations in the gene encoding DHCR7 lead to increased levels of 7-DHC and the neurodevelopmental syndrome Smith-Lemli-Opitz syndrome (SLOS). 7-DHC is used as a biomarker for SLOS screenings. Inhibits human fibroblast sphingomyelinase. Shown to have cytotoxic effects on melanoma cells. In skin, photolysis of 7-DHC by ultraviolet light produces vitamin D3. UV/Vis: lambdamax 271, 282, 293nm.
MDL:
MFCD00003624
Molecular Formula:
C27H44O
Molecular Weight:
384.64
Package Type:
Vial
Product Description:
Precursor of cholesterol and vitamin D3. It is reduced to cholesterol by the enzyme 3beta-hydroxysterol-Delta7-reductase (DHCR7) in the last step of cholesterol biosynthesis. Mutations in the gene encoding DHCR7 lead to increased levels of 7-DHC and the neurodevelopmental syndrome Smith-Lemli-Opitz syndrome (SLOS). 7-DHC is used as a biomarker for SLOS screenings. Inhibits human fibroblast sphingomyelinase. Shown to have cytotoxic effects on melanoma cells. In skin, photolysis of 7-DHC by ultraviolet light produces vitamin D3. UV/Vis: lambdamax 271, 282, 293nm.
Purity:
>95% (HPLC)
SMILES:
[H][C@@]1(CC[C@@]2([H])C3=CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C
Solubility Chemicals:
Soluble in ethanol, chloroform or ethyl acetate. Slightly soluble in DMSO. Insoluble in water.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) T. Okano, et al.; J. Nutr. Sci. Vitaminol. 24, 47 (1978) | (2) J.c. Maziere, et al.; BBRC 100, 1299 (1981) | (3) Q. Xiong, et al.; Chem. Phys. Lipids 115, 1 (2002) | (4) A. Valencia, et al.; Free. Radic. Biol. Med. 41, 1704 (2006) | (5) M. Gelzo, et al.; Free. Radic. Biol. Med. 70, 129 (2014) | (6) W. Liu, et al.; J. Lipid. Res. 55, 329 (2014)
Related Products
| Product Name | Product Code | Supplier | 7beta-Hydroxy-cholesteryl-bishemisuccinate-diethanolamine salt | CDX-H0123 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|