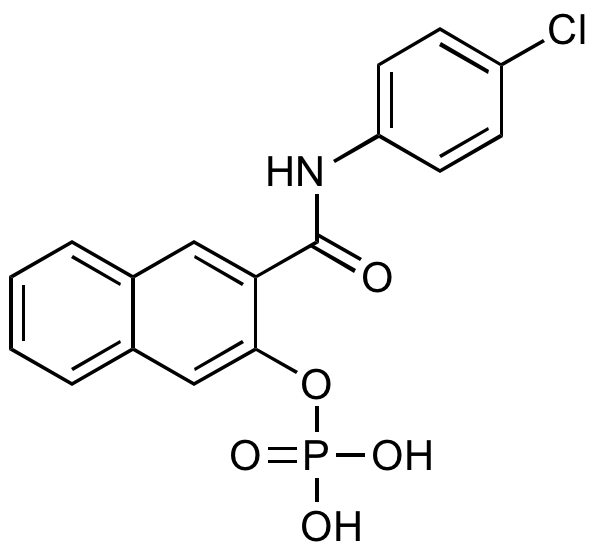
Naphthol AS-E phosphate
| Code | Size | Price |
|---|
| CDX-N0125-M500 | 500 mg | £121.00 |
Quantity:
| CDX-N0125-G001 | 1 g | £200.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
N-(4-Chlorophenyl)-3-(phosphonooxy)naphthalene-2-carboxamide; KG-501; NASEP
Appearance:
Beige powder.
CAS:
18228-17-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C17H13ClNO5P/c18-13-5-7-14(8-6-13)19-17(20)15-9-11-3-1-2-4-12(11)10-16(15)24-25(21,22)23/h1-10H,(H,19,20)(H2,21,22,23)
InChiKey:
RQAQWBFHPMSXKR-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 18228-17-6. Formula: C17H13ClNO5P. MW: 377.72. Synthetic. Histochemical substrate for alkaline phosphatases. Aldolase inhibitor. Small molecule inhibitor. Anticancer compound that directly targets the KIX domain of CBP, resulting in a disrupted CREB-CBP complex, inhibited CREB-target gene induction and inhibited IL-1beta-mediated angiogenic activity in cancer cell lines. Inhibits activity of transcription factor Myb by blocking interaction with KIX domain of coactivator p300 in human leukemia cells. Interferes with the Myb?KIX interaction in vitro and inhibits Myb activity in vivo. Suppresses the expression of Myb target genes and induces myeloid differentiation and apoptosis.
MDL:
MFCD00042718
Molecular Formula:
C17H13ClNO5P
Molecular Weight:
377.72
Package Type:
Vial
Product Description:
Histochemical substrate for alkaline phosphatases. Aldolase inhibitor. Small molecule inhibitor. Anticancer compound that directly targets the KIX domain of CBP, resulting in a disrupted CREB-CBP complex, inhibited CREB-target gene induction and inhibited IL-1beta-mediated angiogenic activity in cancer cell lines. Inhibits activity of transcription factor Myb by blocking interaction with KIX domain of coactivator p300 in human leukemia cells. Interferes with the Myb?KIX interaction in vitro and inhibits Myb activity in vivo. Suppresses the expression of Myb target genes and induces myeloid differentiation and apoptosis.
Purity:
>99% (HPLC)
SMILES:
OP(O)(=O)OC1=CC2=CC=CC=C2C=C1C(=O)NC1=CC=C(Cl)C=C1
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Enzyme Substrates
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) Z. Lojda, et al.; Histochemie 11, 13 (1967) | (2) J.L. Best, et al.; PNAS 101, 17622 (2004) | (3) M. St-Jean, et al.; J. Biol. Chem. 282, 14309 (2007) | (4) B.X. Li & X. Xiao, et al.; ChemBioChem 10, 2721 (2009) | (5) J.W. Lee, et al.; PLoS One 10, e0122628 (2015) | (6) S. Uttarkar, et al.; Mol. Cancer Ther. 14, 1276 (2015)
Related Products
| Product Name | Product Code | Supplier | Nitrotetrazolium blue chloride | CDX-N0009 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|