PTACH
| Code | Size | Price |
|---|
| CDX-P0063-M005 | 5 mg | £126.00 |
Quantity:
| CDX-P0063-M025 | 25 mg | £474.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NCH 51; Cpd 51; S-[6-(4-Phenyl-2-thiazolylcarbamoyl)hexyl] thioisobutyrate
Appearance:
White crystals.
CAS:
848354-66-5
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS05
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H318, H413
InChi:
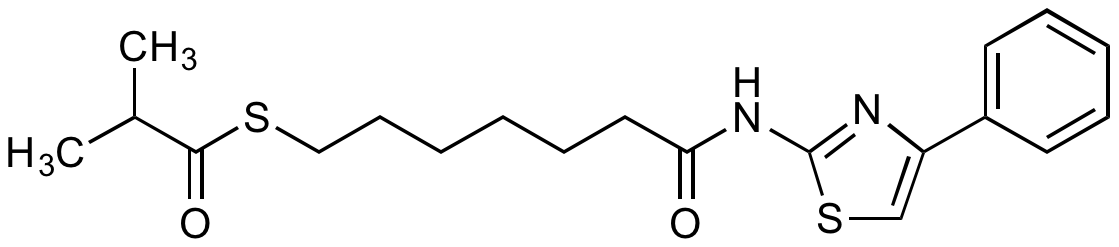
InChI=1S/C20H26N2O2S2/c1-15(2)19(24)25-13-9-4-3-8-12-18(23)22-20-21-17(14-26-20)16-10-6-5-7-11-16/h5-7,10-11,14-15H,3-4,8-9,12-13H2,1-2H3,(H,21,22,23)
InChiKey:
MDYDGUOQFUQOGE-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 848354-66-5. Formula: C20H26N2O2S2. MW: 390.56. Synthetic. Non-hydroxamate HDAC inhibitor (HDACi) (IC50: 32, 48 and 41nM for HDAC4, HDAC1 and HDAC6, respectively). Cell-permeable prodrug that is intracellularly converted to a potent HDAC inhibitor. Predicted to exhibit a similar HDAC binding mode as that of SAHA, interacting with the active-site zinc targeting group. Shown to exhibit comparable antiproliferative and apoptotic activity as SAHA against various cancer cell lines. Inhibits growth of various cancer cells in vitro (EC50 = 1.1 - 9.1mM). Also reactivates latent HIV-1 gene expression.
MDL:
MFCD08705329
Molecular Formula:
C20H26N2O2S2
Molecular Weight:
390.56
Package Type:
Vial
Precautions:
P280, P305, P351, P338
Product Description:
Non-hydroxamate HDAC inhibitor (HDACi) (IC50: 32, 48 and 41nM for HDAC4, HDAC1 and HDAC6, respectively). Cell-permeable prodrug that is intracellularly converted to a potent HDAC inhibitor. Predicted to exhibit a similar HDAC binding mode as that of SAHA, interacting with the active-site zinc targeting group. Shown to exhibit comparable antiproliferative and apoptotic activity as SAHA against various cancer cell lines. Inhibits growth of various cancer cells in vitro (EC50 = 1.1 - 9.1µM). Also reactivates latent HIV-1 gene expression.
Purity:
>95% (HPLC)
Signal word:
Danger
SMILES:
CC(C)C(=O)SCCCCCCC(=O)NC1=NC(=CS1)C1=CC=CC=C1
Solubility Chemicals:
Soluble in DMSO (25mg/ml).
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) T. Suzuki, et al.; J. Med. Chem. 48, 1019 (2005) | (2) T. Sanda, et al.; Leukemia 21, 2344 (2007) | (3) T. Suzuki, et al.; Bioorg. Med. Chem. Lett. 17, 1558 (2007) | (4) A.F. Victoriano, et al.; FEBS Lett. 585, 1103 (2011)
Related Products
| Product Name | Product Code | Supplier |
|---|