ZnAF-2F Solution
| Code | Size | Price |
|---|
| CDX-Z0510-M001 | 1 mg | £487.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
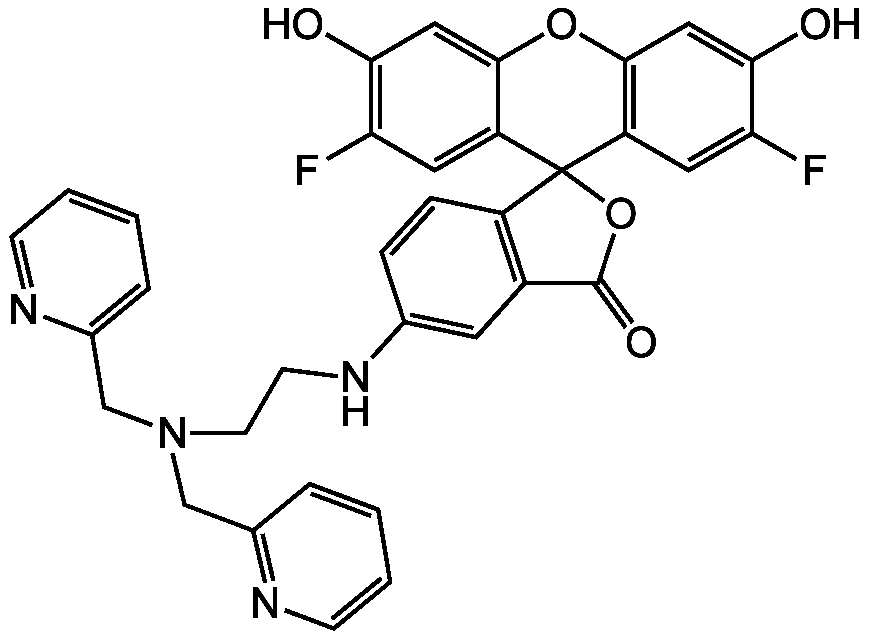
6-{2-[Bis(2-pyridylmethyl)amino]ethylamino}-2',7'-difluorofluorescein
Appearance:
Liquid.
CAS:
443302-09-8
Concentration:
5mM in DMSO (1mg in 0.33ml DMSO)
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.
InChi:
InChI=1S/C34H26F2N4O5/c35-27-14-25-31(16-29(27)41)44-32-17-30(42)28(36)15-26(32)34(25)24-8-7-20(13-23(24)33(43)45-34)39-11-12-40(18-21-5-1-3-9-37-21)19-22-6-2-4-10-38-22/h1-10,13-17,39,41-42H,11-12,18-19H2
InChiKey:
VRUAOMSCYBFMTB-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 443302-09-8. Formula: C34H26F2N4O5. MW: 608.59. Synthetic. ZnAF-1F and ZnAF-2F do not fluoresce in the presence of other biologically important cations such as Ca2+ and Mg2+, and are insensitive to change of pH. The complexes with Zn2+ of previously developed ZnAFs, ZnAF-1, and ZnAF-2 decrease in fluorescence intensity below pH 7.0 owing to protonation of the phenolic hydroxyl group of fluorescein, whose pKa value is 6.2. On the other hand, the Zn2+ complexes of ZnAF-1F and ZnAF-2F emit stable fluorescence around neutral and slightly acidic conditions because the pKa values are shifted to 4.9 by substitution of electron-withdrawing fluorine at the ortho position of the phenolic hydroxyl group. Spectral data: lambdaex 492nm; lambdaem 517nm in PBS.
MDL:
MFCD28385818
Molecular Formula:
C34H26F2N4O5
Molecular Weight:
608.59
Package Type:
Vial
Product Description:
ZnAF-1F and ZnAF-2F do not fluoresce in the presence of other biologically important cations such as Ca2+ and Mg2+, and are insensitive to change of pH. The complexes with Zn2+ of previously developed ZnAFs, ZnAF-1, and ZnAF-2 decrease in fluorescence intensity below pH 7.0 owing to protonation of the phenolic hydroxyl group of fluorescein, whose pKa value is 6.2. On the other hand, the Zn2+ complexes of ZnAF-1F and ZnAF-2F emit stable fluorescence around neutral and slightly acidic conditions because the pKa values are shifted to 4.9 by substitution of electron-withdrawing fluorine at the ortho position of the phenolic hydroxyl group. Spectral data: lambdaex 492nm; lambdaem 517nm in PBS.
Purity:
>95% (HPCE)
SMILES:
OC1=CC2=C(C=C1F)C1(OC(=O)C3=C1C=CC(NCCN(CC1=CC=CC=N1)CC1=NC=CC=C1)=C3)C1=CC(F)=C(O)C=C1O2
Solubility Chemicals:
Soluble in DMSO.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) T. Hirano et al. JACS 124, 6555 (2002)