T-2 Toxin
| Code | Size | Price |
|---|
| AG-CN2-0473-M001 | 1 mg | £80.00 |
Quantity:
| AG-CN2-0473-M005 | 5 mg | £290.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short term: +4°C, Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Epoxytrichothecene; Fusariotoxin T-2; Insariotoxin; Mycotoxin T-2; NSC 138780
Appearance:
White solid.
CAS:
21259-20-1
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Keep cool and dry.Protect from light.
Hazards:
H300, H302, H312, H332, H310, H330, H315, H319
InChi:
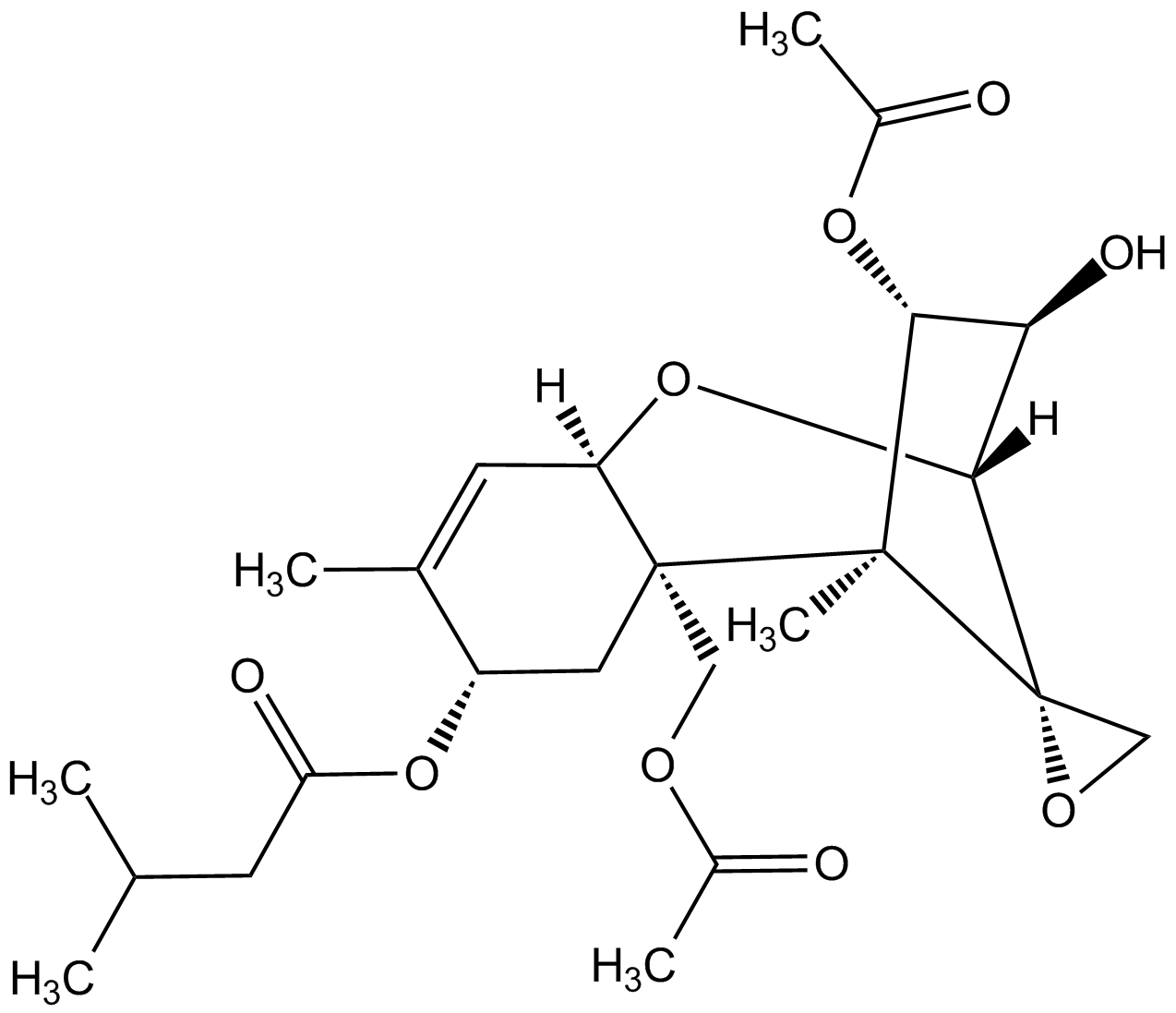
InChI=1S/C24H34O9/c1-12(2)7-18(27)32-16-9-23(10-29-14(4)25)17(8-13(16)3)33-21-19(28)20(31-15(5)26)22(23,6)24(21)11-30-24/h8,12,16-17,19-21,28H,7,9-11H2,1-6H3/t16-,17+,19+,20-,21+,22+,23+,24-/m0/s1
InChiKey:
BXFOFFBJRFZBQZ-CNLOIQPPSA-N
Long Description:
Chemical. CAS: 21259-20-1. Formula: C24H34O9. MW: 466.5. Isolated from fungus Fusarium tricinctum. Common trichothecene mycotoxin, which can infect grain crops causing alimentary toxic aleukia in humans and animals. Inhibits DNA and RNA synthesis in vivo and in vitro and can induce apoptosis. In vivo the compound rapidly metabolizes to HT-2 mycotoxin (a major metabolite). Inhibits protein synthesis through its high binding affinity to peptidyl transferase, which is an integral part of the 60S ribosomal subunit, resulting in activation of JNK/p38 MAPKs. Interferes with the metabolism of membrane phospholipids and increases liver lipid peroxides. Increases blood-brain barrier permeability and inhibits monoamine oxidase activity in brain. Reactive oxygen species (ROS) inducer.
MDL:
MFCD00871255
Molecular Formula:
C24H34O9
Molecular Weight:
466.5
Package Type:
Vial
PG:
I
Precautions:
P260, P280, P301, P310, P302, P352, P304, P340, P305, P351, P338, P405
Product Description:
Common trichothecene mycotoxin, which can infect grain crops causing alimentary toxic aleukia in humans and animals. Inhibits DNA and RNA synthesis in vivo and in vitro and can induce apoptosis. In vivo the compound rapidly metabolizes to HT-2 mycotoxin (a major metabolite). Inhibits protein synthesis through its high binding affinity to peptidyl transferase, which is an integral part of the 60S ribosomal subunit, resulting in activation of JNK/p38 MAPKs. Interferes with the metabolism of membrane phospholipids and increases liver lipid peroxides. Increases blood-brain barrier permeability and inhibits monoamine oxidase activity in brain. Reactive oxygen species (ROS) inducer.
Purity:
>98% (TLC)
Signal word:
Danger
SMILES:
[H][C@@]12O[C@]3([H])C=C(C)[C@H](C[C@]3(COC(C)=O)[C@@](C)([C@H](OC(C)=O)[C@H]1O)[C@]21CO1)OC(=O)CC(C)C
Solubility Chemicals:
Soluble in DMSO (30mg/ml), ethanol (20mg/ml) or dichloromethane.
Source / Host:
Isolated from fungus Fusarium tricinctum.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352211
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
The structures of toxins from two strains of Fusarium tricinctum: J.R. Bamburg, et al.; Tetrahedron 24, 3329 (1968) | Measurement of the stability of T-2 toxin in aqueous solution: J. Mary, et al.; Chem. Res. Tox. 6, 524 (1993) | T-2 toxin-induced apoptosis in lymphoid organs of mice: J. Shinozuka, et al.; Exp. Toxicol. Pathol. 49, 387 (1997) | Apoptotic cellular damage in mice after T-2 toxin-induced acute toxicosis: T. Ihara, et al.; Nat. Toxins 5, 141 (1997) | T-2 toxin-induced apoptosis in hematopoietic tissues of mice: J. Shinozuka, et al.; Toxicol. Pathol. 26, 674 (1998) | Effect of T-2 toxin on blood-brain barrier permeability monoamine oxidase activity and protein synthesis in rats: J. Wang, et al.; Food Chem. Toxicol. 36, 955 (1998) | Cytotoxicity and related effects of T-2 toxin on cultured Vero cells: C. Bouaziz, et al.; Toxicon 48, 343 (2006) | Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways: K. Doi & K. Uetsuka; Int. J. Mol. Sci. 12, 5213 (2011) | T-2 toxin, a trichothecene mycotoxin: review of toxicity, metabolism, and analytical methods: Y. Li, et al.; J. Agric. Food Chem. 59, 3441 (2011) | The fungal T-2 toxin alters the activation of primary macrophages induced by TLR-agonists resulting in a decrease of the inflammatory response in the pig: J. Seeboth, et al.; Vet. Res. 43, 1 (2012) | Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update: Q.H. Wu, et al.; Arch. Toxicol. 88, 1309 (2014)
Related Products
| Product Name | Product Code | Supplier | Fuscin | AG-CN2-0138 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neoxaline | AG-CN2-0154 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||