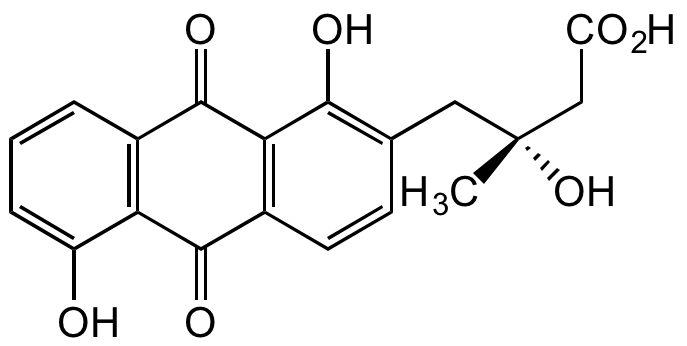
Fridamycin E
| Code | Size | Price |
|---|
| BVT-0472-M001 | 1 mg | £105.00 |
Quantity:
| BVT-0472-M005 | 5 mg | £300.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short Term: +4°C Long Term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
9,10-Dihydro-beta,1,5-trihydroxy-beta-methyl-9,10-dioxo-2-anthracenebutanoic acid
Appearance:
Yellow to orange crystals.
CAS:
116120-54-8
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light.
InChi:
InChI=1S/C19H16O7/c1-19(26,8-13(21)22)7-9-5-6-11-15(16(9)23)18(25)10-3-2-4-12(20)14(10)17(11)24/h2-6,20,23,26H,7-8H2,1H3,(H,21,22)/t19-/m1/s1
InChiKey:
MVEDBMGRQONWSQ-LJQANCHMSA-N
Long Description:
Chemical. CAS: 116120-54-8. Formula: C19H16O7. MW: 356.3. Isolated from Streptomyces parvulus. Anthraquinone derivative derived from an angucyclinone precursor. Antibiotic. Antibacterial agent.
MDL:
N/A
Molecular Formula:
C19H16O7
Molecular Weight:
356.3
Package Type:
Plastic Vial
Product Description:
Anthraquinone derivative derived from an angucyclinone precursor. Antibiotic. Antibacterial agent.
Purity:
>98% (HPLC, NMR)
SMILES:
C[C@](O)(CC(O)=O)CC1=C(O)C2=C(C=C1)C(=O)C1=C(C=CC=C1O)C2=O
Solubility Chemicals:
Soluble in DMSO. Sparingly soluble in methanol or acetone.
Source / Host:
Isolated from Streptomyces parvulus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
Fridamycine, Anthracyclin-analoge Antibiotica: P. Knicke; Ph. D. Thesis Univ. Goettingen (1984) | Synthesis of rac- and ent-fridamycin E: K. Krohn & W. Baltus; Tetrahedron 44, 49 (1988) | Synthetic anthracyclines from anthraquinones: R. Cambie, et al.; Austr. J. Chem. 45, 483 (1992) | C-Aryl glycosides via tandem intramolecular benzyne-furan cycloadditions. Total synthesis of vineomycine B2 methyl ester: C.-L. Chen, et al.; JACS 128, 13696 (2006) | A chemoenzymatic, enantioconvergent, asymmetric total synthesis of (R)-fridamycin E: B.J. Ueberbacher, et al.; Eur. J. Org. Chem. 7, 1266 (2005) | Gephyromycin, the first bridged angucyclinone, from streptomyces griseus strain NTK 14: G. Bringmann, et al.; Phytochemistry 66, 1366 (2005) | De novo asymmetric synthesis of fridamycin E: Q. Chen, et al.; Org. Lett. 13, 6592 (2011) | Angucyclines from an insect-derived actinobacterium Amycolatopsis sp. HCa1 and their cytotoxic activity: Z.K. Guo, et al.; Bioorg. Med. Chem. Lett. 22, 7490 (2012)