Genipin
Product Code:
AG-CN2-0481
AG-CN2-0481
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short Tem: +4°C, Long Term: -20°C
Short Tem: +4°C, Long Term: -20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CN2-0481-M025 | 25 mg | £60.00 |
Quantity:
| AG-CN2-0481-M100 | 100 mg | £160.00 |
Quantity:
| AG-CN2-0481-M500 | 500 mg | £400.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
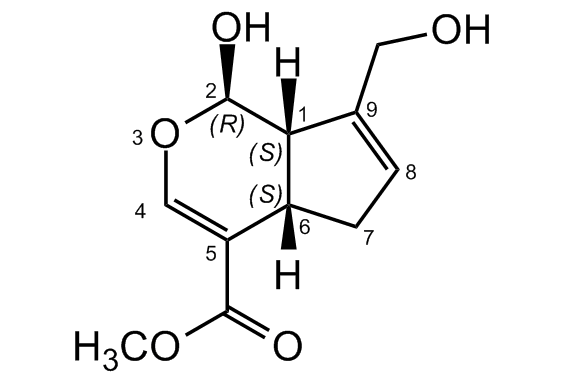
(+)-Genipin; Methyl (1S,2R,6S)-2-hydroxy-9-(hydroxymethyl)-3-oxabicyclo[4.3.0]nona-4,8-diene-5-carboxylate
Appearance:
White to off-white solid.
CAS:
6902-77-8
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Keep cool and dry.Protect from light.
Hazards:
H301
InChi:
InChI=1S/C11H14O5/c1-15-10(13)8-5-16-11(14)9-6(4-12)2-3-7(8)9/h2,5,7,9,11-12,14H,3-4H2,1H3/t7-,9-,11-/m1/s1
InChiKey:
AZKVWQKMDGGDSV-BCMRRPTOSA-N
Long Description:
Chemical. CAS: 6902-77-8. Formula: C11H14O5. MW: 226.2. Isolated from Gardenia jasminoides Ellis fruits. Cell permeable inhibitor of uncoupling protein 2 (UCP2). Increases glucose-stimulated insulin secretion, mitochondrial membrane potential and ATP levels in pancreatic island cells. Anticancer, antimetastatic and anti-angiogenic agent. Increases Bax/Bcl-2 ratio and induces PARP cleavage and caspase-3- and caspase-9-mediated apoptosis in non-small cell lung cancer (NSCLC) cells. Sensitizes multidrug-resistant cancer cells to cytotoxic agents through UCP2 inhibition. Suppresses the tumor promoting function of UCP2 in a cellular model of Warburg effect. Anti-inflammatory agent. Upregulates nNOS (neuronal nitric oxide synthase/NOS I) and decreases acute inflammation through inhibition of LPS-induced NF-kappaB activity. Inhibits toll-like receptor (TLR) signaling and the production of pro-inflammatory cytokines, preventing sepsis and increasing survival in animal models of infection. Modulates NLRP3 inflammasome activation and ATP- or H2O2-mediated IL-1beta release. Inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Antiviral compound. Chemical promoter of Epstein-Barr virus (EBV) lytic cycle activation and suppressor of EBV infection. Shown to have wound healing, neuroprotective, antidepressant, anti-osteoporotic and antioxidative activities. Protein, collagen, gelatin and chitosan natural cross-linking agent. 5,000-10,000 times less cytotoxic than any other crosslinking agents, with favorable properties of use in the range of pH 7.4-8.5 and temperatures between 25-45°C. Shown to be useful in the construction of novel drug delivery systems, as the raw material for gardenia blue pigment preparation and as the intermediate for alkaloid syntheses. Produces blue colored pigments by binding to amino acids. Simple, safe, sensitive and stable alternative for colorimetric determination of amino acids. Shown in forensic research to be a novel fingerprint reagent with colorimetric and fluorogenic activity.
MDL:
MFCD00888600
Molecular Formula:
C11H14O5
Molecular Weight:
226.2
Package Type:
Vial
PG:
III
Precautions:
P301, P310
Product Description:
Cell permeable inhibitor of uncoupling protein 2 (UCP2). Increases glucose-stimulated insulin secretion, mitochondrial membrane potential and ATP levels in pancreatic island cells. Anticancer, antimetastatic and anti-angiogenic agent. Increases Bax/Bcl-2 ratio and induces PARP cleavage and caspase-3- and caspase-9-mediated apoptosis in non-small cell lung cancer (NSCLC) cells. Sensitizes multidrug-resistant cancer cells to cytotoxic agents through UCP2 inhibition. Suppresses the tumor promoting function of UCP2 in a cellular model of Warburg effect. Anti-inflammatory agent. Upregulates nNOS (neuronal nitric oxide synthase/NOS I) and decreases acute inflammation through inhibition of LPS-induced NF-kappaB activity. Inhibits toll-like receptor (TLR) signaling and the production of pro-inflammatory cytokines, preventing sepsis and increasing survival in animal models of infection. Modulates NLRP3 inflammasome activation and ATP- or H2O2-mediated IL-1beta release. Inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Antiviral compound. Chemical promoter of Epstein-Barr virus (EBV) lytic cycle activation and suppressor of EBV infection. Shown to have wound healing, neuroprotective, antidepressant, anti-osteoporotic and antioxidative activities. Protein, collagen, gelatin and chitosan natural cross-linking agent. 5,000-10,000 times less cytotoxic than any other crosslinking agents, with favorable properties of use in the range of pH 7.4-8.5 and temperatures between 25-45°C. Shown to be useful in the construction of novel drug delivery systems, as the raw material for gardenia blue pigment preparation and as the intermediate for alkaloid syntheses. Produces blue colored pigments by binding to amino acids. Simple, safe, sensitive and stable alternative for colorimetric determination of amino acids. Shown in forensic research to be a novel fingerprint reagent with colorimetric and fluorogenic activity.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
[H][C@]12CC=C(CO)[C@@]1([H])[C@H](O)OC=C2C(=O)OC
Solubility Chemicals:
Soluble in DMSO (50mg/ml), ethanol (5mg/ml), methanol or acetone. Slightly soluble in water.
Source / Host:
Isolated from Gardenia jasminoides Ellis fruits.
Transportation:
Excepted Quantity
UN Nummer:
UN 2811
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352211
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Naturally occurring oxygen heterocyclics. IX. Isolation and characterization of genipin: C. Djerassi, et al.; J. Org. Chem. 25, 2174 (1960) | Prevention of the neurotoxicity of the amyloid beta protein by genipin: M. Yamazaki, et al.; Biol. Pharm. Bull. 24, 1454 (2001) | Colorimetric determination of amino acids using genipin from Gardenia jasminoides: S.W. Lee, et al.; Anal. Chim. Acta 480, 267 (2003) | Genipin-a novel fingerprint reagent with colorimetric and fluorogenic activity: J. Almog, et al.; J. Forensic Sci. 49, 255 (2004) | Cyclic GMP-dependent neurite outgrowth by genipin and nerve growth factor in PC12h cells: M. Yamazaki, et al.; Eur. J. Pharmacol. 488, 35 (2004) | Antiinflammatory effects of genipin, an active principle of gardenia: H.J. Koo, et al.; Eur. J. Pharmacol. 495, 201 (2004) | Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets: C.Y. Zhang, et al.; Cell Metab. 3, 417 (2006) | Genipin exhibits neurotrophic effects through a common signaling pathway in nitric oxide synthase-expressing cells: M. Yamazaki & K. Chiba; Eur. J. Pharmacol. 581, 255 (2008) | Neuroprotective action of genipin on tunicamycin-induced cytotoxicity in neuro2a cells: M. Tanaka, et al.; Biol. Pharm. Bull. 32, 1220 (2009) | Antidepressant-like effect of genipin in mice: J.S. Tian, et al.; Neurosci. Lett. 479, 236 (2010) | Genipin induced apoptosis associated with activation of the c-Jun NH2-terminal kinase and p53 protein in HeLa cells: H. Cao, et al.; Biol. Pharm. Bull. 33, 1343 (2010) | Genipin-induced inhibition of uncoupling protein-2 sensitizes drug-resistant cancer cells to cytotoxic agents: R.J. Mailloux, et al.; PLoS One 5, e13289 (2010) | Cellular model of Warburg effect identifies tumor promoting function of UCP2 in breast cancer and its suppression by genipin: V. Ayyasamy, et al.; PLoS One 6, e24792 (2011) | Genipin attenuates sepsis by inhibiting Toll-like receptor signalling: T.H. Kim, et al.; Mol. Med. 18, 455 (2012) | Up-regulation of TIMP-1 by genipin inhibits MMP-2 activities and suppresses the metastatic potential of human hepatocellular carcinoma: N. Wang, et al.; PLoS One 7, e46318 (2012) | Genipin - the natural water soluble cross-linking agent and its importance in the modified drug delivery systems: an overview: B. Manickam, et al.; Curr. Drug Deliv. 11, 139 (2014) (Review) | Genipin inhibits RANKL-induced osteoclast differentiation through proteasome-mediated degradation of c-Fos protein and suppression of NF-kappaB activation: C.H. Lee, et al.; J. Pharmacol. Sci. 124, 344 (2014) | Genipin as a novel chemical activator of EBV lytic cycle: M. Son, et al.; J. Microbiol. 53, 155 (2015) | Genipin suppresses NLRP3 inflammasome activation through uncoupling protein-2: V. Rajanbabu, et al.; Cell Immunol. 297, 40 (2015) | Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression: S.X. Yu, et al.; Sci. Rep. 5, 17935 (2015)