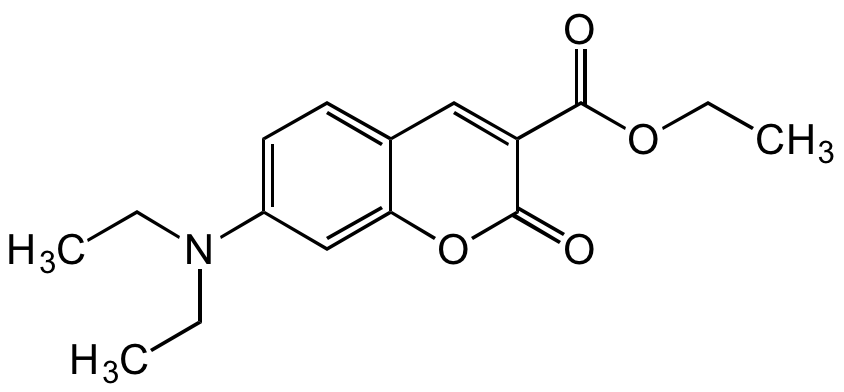
7-Diethylaminocoumarin-3-carboxylic acid ethyl ester
| Code | Size | Price |
|---|
| CDX-D0126-G001 | 1 g | £121.00 |
Quantity:
| CDX-D0126-G005 | 5 g | £450.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
7-DCCA ethyl ester; Ethyl 7-(diethylamino)coumarin-3-carboxylate; NKX 1253; S0679
Appearance:
Light yellow to green powder.
CAS:
28705-46-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C16H19NO4/c1-4-17(5-2)12-8-7-11-9-13(15(18)20-6-3)16(19)21-14(11)10-12/h7-10H,4-6H2,1-3H3
InChiKey:
MSOLGAJLRIINNF-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 28705-46-6. Formula: C16H19NO4. MW: 289.3. Synthetic. Intermediate for the preparation of fluroescent reagents. Laser and scintillation dye. Fluoride chemosensor. Spectral properties: lambdaex=418nm; lambdaem=461nm, in ethanol. Inhibitor of human monoamine oxidase B (hMAO-B).
MDL:
MFCD00227484
Molecular Formula:
C16H19NO4
Molecular Weight:
289.3
Package Type:
Vial
Product Description:
Intermediate for the preparation of fluroescent reagents. Laser and scintillation dye. Fluoride chemosensor. Spectral properties: lambdaex=418nm; lambdaem=461nm, in ethanol. Inhibitor of human monoamine oxidase B (hMAO-B).
Purity:
>98% (HPLC)
SMILES:
O=C1C(C(OCC)=O)=CC2=CC=C(N(CC)CC)C=C2O1
Solubility Chemicals:
Soluble in methanol.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C.
References
(1) N.R. Ayyangar, et al.; Dyes Pigments 16, 197 (1991) | (2) H. Takechi, et al.; Chem. Pharm. Bull. 48, 1702 (2000) | (3) J.E.T. Corrie & V.R.N. Munasinghe; J. Heterocyclic Chem. 37, 1447 (2000) | (4) T.-H. Kim & T.M. Swager; Angew. Chem. Int. Ed. 42, 4803 (2003) | (5) X. Li, et al.; Z. Kristallogr. NCS 224, 593 (2009) | (6) D. Secci, et al.; Eur. J. Med. Chem. 46, 4846 (2011)