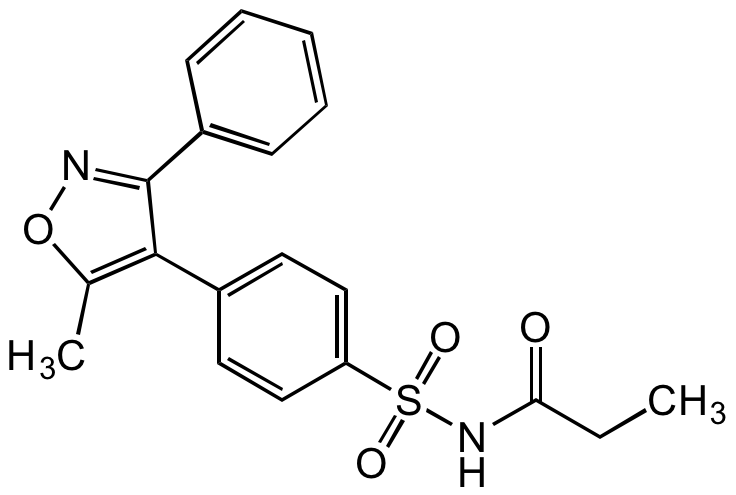
Parecoxib
| Code | Size | Price |
|---|
| CDX-P0167-M025 | 25 mg | £169.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
N-[[4-(5-Methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]propanamide; Dynastat; SC-69124
Appearance:
White powder.
CAS:
198470-84-7
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS08
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H361d, H373
InChi:
InChI=1S/C19H18N2O4S/c1-3-17(22)21-26(23,24)16-11-9-14(10-12-16)18-13(2)25-20-19(18)15-7-5-4-6-8-15/h4-12H,3H2,1-2H3,(H,21,22)
InChiKey:
TZRHLKRLEZJVIJ-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 198470-84-7. Formula: C19H18N2O4S. MW: 370.4. Synthetic. Potent and selective COX-2 inhibitor. Water-soluble amide prodrug of the cyclooxygenase II (COX-2) selective, non-steroidal anti-inflammatory drug (NSAID) valdecoxib. Has anti-inflammatory, analgesic, and antipyretic activities. Upon administration, parecoxib is hydrolyzed by hepatic carboxyesterases to its active form, valdecoxib.
MDL:
MFCD08141856
Molecular Formula:
C19H18N2O4S
Molecular Weight:
370.4
Package Type:
Vial
Precautions:
P281
Product Description:
Potent and selective COX-2 inhibitor. Water-soluble amide prodrug of the cyclooxygenase II (COX-2) selective, non-steroidal anti-inflammatory drug (NSAID) valdecoxib. Has anti-inflammatory, analgesic, and antipyretic activities. Upon administration, parecoxib is hydrolyzed by hepatic carboxyesterases to its active form, valdecoxib.
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
O=C(CC)NS(=O)(C1=CC=C(C2=C(C)ON=C2C3=CC=CC=C3)C=C1)=O
Solubility Chemicals:
Soluble in water.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) J.J. Talley, et al.; J. Med. Chem. 43, 1661 (2000) | (2) S.S. Padi, et al.; Eur. J. Pharmacol. 491, 69 (2004) | (3) A.B. Reksidler, et al.; Eur. J. Pharmacol. 560, 163 (2007) | (4) A. Abbate, et al.; J. Cardiovasc. Pharmacol. 49, 416 (2007) | (5) H. Schroeder, et al.; Neurochem. Int. 58, 9 (2011) | (6) Z. Ye, et al.; Neurochem. Res. 37, 321 (2012) | (7) S.J.Chong, et al.; Int. J. Inflam. 2014, 972645 (2014)