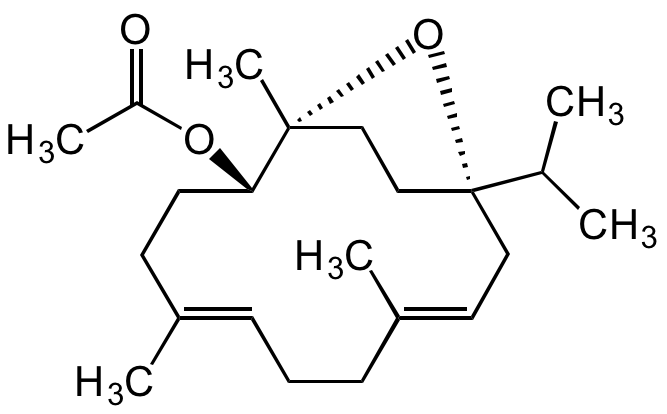
Incensol acetate
Product Code: AG-CN2-0484
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0484-M001 | 1 mg | £135.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Storage:
Short Term: 4°C Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(+)-Incensol acetate; (+)-Incensole acetate; (1R,2S,5E,9E,12S)-(9Cl)-1,5,9-Trimethyl-12-(1-methylethyl)-15-oxabicyclo[10.2.1]pentadeca-5,9-dien-2-ol acetate
Appearance:
Oil.
CAS:
34701-53-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C22H36O3/c1-16(2)22-13-12-18(4)9-7-8-17(3)10-11-20(24-19(5)23)21(6,25-22)14-15-22/h8,12,16,20H,7,9-11,13-15H2,1-6H3/b17-8+,18-12+/t20-,21+,22+/m0/s1
InChiKey:
HVBACKJYWZTKCA-XSLBTUIJSA-N
Long Description:
Chemical. CAS: 34701-53-6. Formula: C22H36O3. MW: 348.5. Semisynthetic from incensol (isolated from Boswellia papyrifera). Component of the frankincense essential oil. Activator of transient receptor potential vanilloid 3 (TRPV3), a heat-sensitive ion channel involved in skin heat sensitivity, thermoregulation and neurological activities. Less powerful TRPV3 agonist than incensol (Prod. No. AG-CN2-0482). Shows in vivo neuroprotective properties. Causes anxiolytic-like and antidepressive-like behavioral effects in wild-type (WT) mice. Anti-inflammatory agent. Shown to have COX-1 and COX-2 inhibitory activity and cytotoxic activity against selected cancer cell lines.
MDL:
MFCD29904535
Molecular Formula:
C22H36O3
Molecular Weight:
348.5
Package Type:
Vial
Product Description:
Component of the frankincense essential oil. Activator of transient receptor potential vanilloid 3 (TRPV3), a heat-sensitive ion channel involved in skin heat sensitivity, thermoregulation and neurological activities. Less powerful TRPV3 agonist than incensol (Prod. No. AG-CN2-0482). Shows in vivo neuroprotective properties. Causes anxiolytic-like and antidepressive-like behavioral effects in wild-type (WT) mice. Anti-inflammatory agent. Shown to have COX-1 and COX-2 inhibitory activity and cytotoxic activity against selected cancer cell lines.
Purity:
>95% (HPLC)
SMILES:
C/C1=CCC/C(C)=C/C[C@]2(C(C)C)CC[C@@](C)(O2)[C@@H](OC(C)=O)CC1
Solubility Chemicals:
Soluble in DMSO, dichloromethane or ethyl ether. Insoluble in water. Almost insoluble in ethanol or methanol.
Source / Host:
Semisynthetic from incensol (isolated from Boswellia papyrifera).
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352211
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Cancer chemopreventive effects and cytotoxic activities of the triterpene acids from the resin of Boswellia carteri: T. Akihisa, et al.; Biol. Pharm. Bull. 29, 1976 (2006) | Incensole acetate, a novel anti-inflammatory compound isolated from Boswellia resin, inhibits nuclear factor-kappa B activation: A. Moussaieff, et al.; Mol. Pharmacol. 72, 1657 (2007) | Incensole acetate: a novel neuroprotective agent isolated from Boswellia carterii: A. Moussaieff, et al.; J. Cereb. Blood Flow Metab. 28, 1341 (2008) | Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain: A. Moussaieff, et al.; FASEB J. 22, 3024 (2008) | Protective effects of incensole acetate on cerebral ischemic injury: A. Moussaieff, et al.; Brain Res. 1443, 89 (2012) | Incensole acetate reduces depressive-like behavior and modulates hippocampal BDNF and CRF expression of submissive animals: A. Moussaieff, et al.; J. Psychopharmacol. 26, 1584 (2012) | Major constituents of Boswellia carteri resin exhibit cyclooxygenase enzyme inhibition and antiproliferative activity: S.I. Ali, et al.; Nat. Prod. Commun. 8, 1365 (2013) | Neuroactive and anti-inflammatory frankincense cembranes: A structure-activity study: F. Pollastro, et al.; J. Nat. Prod. 79, 1762 (2016)