CX-4945 . hydrochloride [Silmitasertib . HCl]
| Code | Size | Price |
|---|
| AG-CR1-3629-M001 | 1 mg | £70.00 |
Quantity:
| AG-CR1-3629-M005 | 5 mg | £180.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
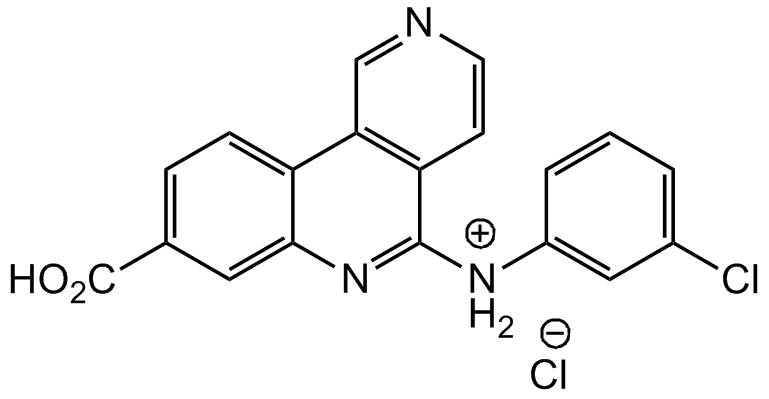
Silmitasertib . HCl; 8-Carboxy-N-(3-chlorophenyl)benzo[c][2,6] naphthyridin-5-aminium chloride
Appearance:
Yellow solid.
CAS:
1009820-21-6 (parent)
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C19H12ClN3O2.ClH/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18;/h1-10H,(H,22,23)(H,24,25);1H
InChiKey:
OBOIGMDUOMSAHW-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1009820-21-6 (free acid). Formula: C19H12ClN3O2 . HCl. MW: 349.8 . 36.5. Synthetic. Orally active, potent and selective ATP-competitive inhibitor of the protein kinase CK2 (IC50=1nM). Anticancer compound. Inhibits proliferation in a panel of cancer cell lines that overexpress CK2. Inhibited migration, blocks survival and induces apoptosis in cancer stem cells, glioblastomas and leukemia cells. Shown to decrease the glucose metabolism in cancer cells. Potent inhibitor of Cdc2-like kinases (Clks) in vitro, consequently interfering with alternative splicing. Potent ATP-competitive inhibitor of DYRK1A (IC50=6.8?nM), involved in neurodegenration-associated diseases. CK2alpha deletion selectively increased M3 muscarinic receptors (M3Rs)-mediated insulin secretion from pancreatic islets. Promoted cAMP-induced thermogenesis in white adipocytes. CK2 inhibition ameliorates diet-induced obesity and insulin resistance in mice in vivo by promoting UCP1-dependent thermogenesis.
MDL:
MFCD13184796
Molecular Formula:
C19H12ClN3O2 . HCl . xH2O
Molecular Weight:
349.8 . 36.5 . x18.0
Package Type:
Vial
Product Description:
Orally active, potent and selective ATP-competitive inhibitor of the protein kinase CK2 (IC50=1nM). Anticancer compound. Inhibits proliferation in a panel of cancer cell lines that overexpress CK2. Inhibited migration, blocks survival and induces apoptosis in cancer stem cells, glioblastomas and leukemia cells. Shown to decrease the glucose metabolism in cancer cells. Potent inhibitor of Cdc2-like kinases (Clks) in vitro, consequently interfering with alternative splicing. Potent ATP-competitive inhibitor of DYRK1A (IC50=6.8nM), DYRK1B (IC50=6.4nM) and DYRK3 (IC50=18nM), involved in neurodegeneration-associated diseases. CK2alpha deletion selectively increased M3 muscarinic receptors (M3Rs)-mediated insulin secretion from pancreatic islets. Promoted cAMP-induced thermogenesis in white adipocytes. CK2 inhibition ameliorates diet-induced obesity and insulin resistance in mice in vivo by promoting UCP1-dependent thermogenesis.
Purity:
>98%
SMILES:
ClC1=CC=CC([NH2+]C2=NC3=CC(C(O)=O)=CC=C3C4=C2C=CN=C4)=C1.[Cl-]
Solubility Chemicals:
Soluble in DMSO (10mg/ml). Insoluble in water.
Transportation:
Non-hazardous
UNSPSC Category:
Protein Kinase Modulators
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy: A. Siddiqui-Jain, et al.; Cancer Res. 70, 10288 (2010) | Structural basis of CX-4945 binding to human protein kinase CK2: A.D. Ferguson, et al.; FEBS Lett. 585, 104 (2011) | Discovery and SAR of 5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer: F. Pierre, et al.; J. Med. Chem. 54, 635 (2011) | Pre-clinical characterization of CX-4945, a potent and selective small molecule inhibitor of CK2 for the treatment of cancer: F. Pierre, et al.; Mol. Cell Biochem. 356, 37 (2011) | CX-4945, a selective inhibitor of casein kinase-2 (CK2), exhibits anti-tumor activity in hematologic malignancies including enhanced activity in chronic lymphocytic leukemia when combined with fludarabine and inhibitors of the B-cell receptor pathway: R.C. Prins, et al.; Leukemia 27, 2094 (2013) | CK2 inhibitor CX4945 induces sequential inactivation of proteins in the signaling pathways related with cell migration and suppresses metastasis of A549 human lung cancer cells: M.J. Ku, et al.; Bioorg. Med. Chem. Lett. 23, 5609 (2013) | Identification of a novel function of CX-4945 as a splicing regulator: H. Kim, et al.; PLos One 9, e94978 (2014) | Activity of the clinical-stage CK2-specific inhibitor CX-4945 against chronic lymphocytic leukemia: L.R. Martins, et al.; Leukemia 28, 179 (2014) | Phosphoproteomics identifies CK2 as a negative regulator of beige adipocyte thermogenesis and energy expenditure: K. Shinoda, et al.; Cell Metab. 22, 997 (2015) | CK2 acts as a potent negative regulator of receptor-mediated insulin release in vitro and in vivo: M. Rossi, et al.; PNAS 112, E6818 (2015) | The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies: H.J. Chon, et al.; Front. Pharmacol. 6, 70 (2015) | A chemical with proven clinical safety rescues Down-syndrome-related phenotypes in through DYRK1A inhibition: H. Kim, et al.; Dis. Model Mech. 9, 839 (2016) | Targeting protein kinase CK2 suppresses bladder cancer cell survival via the glucose metabolic pathway: X. Zhang, et al.; Oncotarget 7, 87361 (2016) | Upregulation of IGF2R evades lysosomal dysfunction-induced apoptosis of cervical cancer cells via transport of cathepsins: T. Takeda, et al.; Cell Death Dis. 10, 876 (2019)
Related Products
| Product Name | Product Code | Supplier | CK2 Inhibitor 10 | AG-CR1-3626 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AZD-7762 hydrochloride | SYN-1017 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (+)-JQ1 | SYN-3004 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||