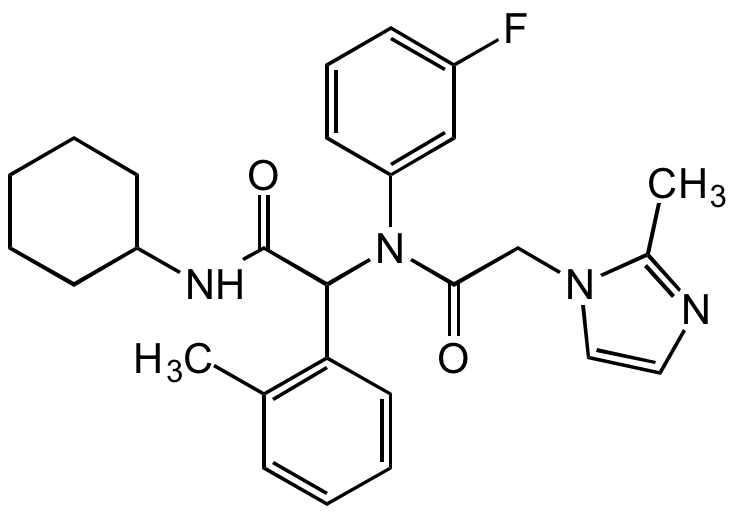
AGI-5198
| Code | Size | Price |
|---|
| AG-CR1-3528-M005 | 5 mg | £80.00 |
Quantity:
| AG-CR1-3528-M025 | 25 mg | £220.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
Short Term: +4°C. Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
N-[2-(Cyclohexylamino)-1-(2-methylphenyl)-2-oxoethyl]-N-(3-fluorophenyl)-2-methyl-1H-imidazole-1-acetamide; IDH-C35
Appearance:
White solid.
CAS:
1355326-35-0
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Keep cool and dry.
Hazards:
H301
InChi:
InChI=1S/C27H31FN4O2/c1-19-9-6-7-14-24(19)26(27(34)30-22-11-4-3-5-12-22)32(23-13-8-10-21(28)17-23)25(33)18-31-16-15-29-20(31)2/h6-10,13-17,22,26H,3-5,11-12,18H2,1-2H3,(H,30,34)FNYGWXSATBUBER-UHFFFAOYSA-N
InChiKey:
FNYGWXSATBUBER-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1355326-35-0. Formula: C27H31FN4O2. MW: 462.6. Synthetic. Potent and selective inhibitor of IDH1 (isocitrate dehydrogenase 1) R132H and R132C mutants in vitro with IC50 values of 0.07 and 0.16 µM, respectively. Does not inhibit wild-type IDH1 or any of the examined IDH2 isoforms (IC50 > 100 µM). Isocitrate dehydrogenases (IDHs) are nicotinamide adenine dinucleotide (NAD+) and NAD phosphate (NADP+)-dependent enzymes in the tricarboxylic acid cycle that catalyze oxidative decarboxylation of isocitrate producing alpha-ketoglutarate (2-OG) and carbon dioxide. IDH1 and IDH2 are mutated in >70% of lower grade gliomas. Shown to have anti-tumor efficacy in the TS603 glioma cell line and to reduce tumor 2-HG production in HT1080 and U87MG cells. Caused 50-60% growth inhibition over a treatment period of three weeks with no affect in the growth of IDH1 wild-type glioma xenografts in R132H-IDH1 glioma xenografts. Under conditions of near complete 2-HG inhibition, induces demethylation of histone H3K9me3 and expression of genes associated with gliogenic differentiation. Useful chemical probe to assess the biological consequences of IDH1 mutations and the potential of IDH1 inhibiton for treating IDH1 mutant tumors. Requires high doses for in vivo activity, but can be used through oral dosing route.
MDL:
MFCD24848688
Molecular Formula:
C27H31FN4O2
Molecular Weight:
462.6
Package Type:
Vial
PG:
III
Precautions:
P264, P301, P310, P405
Product Description:
Potent and selective inhibitor of IDH1 (isocitrate dehydrogenase 1) R132H and R132C mutants in vitro with IC50 values of 0.07 and 0.16µM, respectively. Does not inhibit wild-type IDH1 or any of the examined IDH2 isoforms (IC50>100µM). Isocitrate dehydrogenases (IDHs) are nicotinamide adenine dinucleotide (NAD+) and NAD phosphate (NADP+)-dependent enzymes in the tricarboxylic acid (TCA) cycle that catalyze oxidative decarboxylation of isocitrate producing alpha-ketoglutarate (2-OG) and carbon dioxide. IDH1 and IDH2 are mutated in >70% of lower grade gliomas. Shown to have anti-tumor efficacy in the TS603 glioma cell line and to reduce tumor 2-HG production in HT1080 and U87MG cells. Caused 50-60% growth inhibition over a treatment period of three weeks with no affect in the growth of IDH1 wild-type glioma xenografts in R132H-IDH1 glioma xenografts. Under conditions of near complete 2-HG inhibition, induces demethylation of histone H3K9me3 and expression of genes associated with gliogenic differentiation. Useful chemical probe to assess the biological consequences of IDH1 mutations and the potential of IDH1 inhibition for treating IDH1 mutant tumors. Requires high doses for in vivo activity, but can be used through oral dosing route.
Purity:
>98% (NMR)
Signal word:
Danger
SMILES:
O=C(C(C1=C(C)C=CC=C1)N(C2=CC(F)=CC=C2)C(CN3C=CN=C3C)=O)NC4CCCCC4
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or ethanol (5mg/ml).
Transportation:
Excepted Quantity
UN Nummer:
UN2811
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Cancer-associated IDH1 mutations produce 2-hydroxyglutarate: L. Dang, et al.; Nature 462, 739 (2009) | Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation: P. Koivunen, et al.; Nature 483, 484 (2012) | IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype: S. Turcan, et al. Nature 483, 479 (2012) | Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism: Z.J. Reitman & H. Yan; J. Natl. Cancer Inst. 102, 932 (2010) | Discovery of the first potent inhibitors of mutant IDH1 that lower tumor 2-HG in vivo: J. Popovici-Muller, et al.; ACS Med. Chem. Lett. 3, 850 (2012) | An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells: D. Rohle, et al.; Science 340, 626 (2013)
Related Products
| Product Name | Product Code | Supplier |
|---|