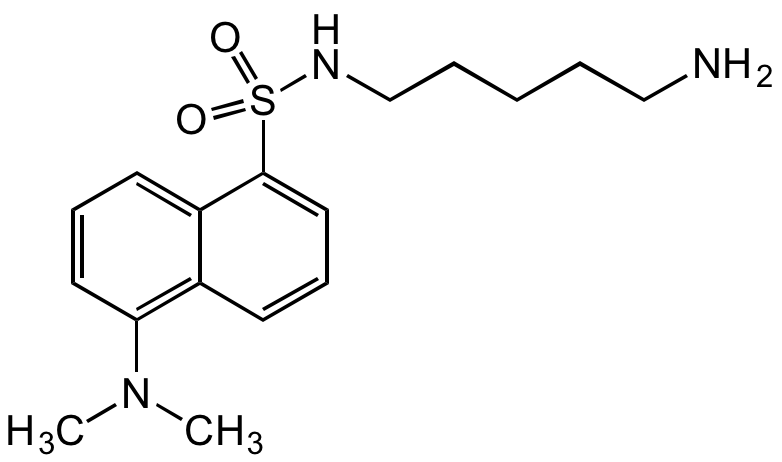
Dansylcadaverine
| Code | Size | Price |
|---|
| CDX-D0189-M250 | 250 mg | £102.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short Term: -20°C . Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
N-(5-Amino?pentyl)-5-di?methyl?amino?naphtha?lene-1-sulfon?amide; N-(Dimethyl?amino?naphtha?lene?sulfonyl)-1,5-pentane?diamine; MDC; Monodansyl cadaverine
Appearance:
Off-white solid.
CAS:
10121-91-2
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C17H25N3O2S/c1-20(2)16-10-6-9-15-14(16)8-7-11-17(15)23(21,22)19-13-5-3-4-12-18/h6-11,19H,3-5,12-13,18H2,1-2H3
InChiKey:
MLEBFEHOJICQQS-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 10121-91-2. Formula: C17H25N3O2S. MW: 335.46. Synthetic. Fluorescent probe for derivatization of aldehydes, ketones and carboxylic acids. Used to prepare small fluorescent biomolecules via amidation or reductive amination. Used to monitor autophagy. Functions by marking the autophagosomes, which allows researchers to follow the molecular components of the process throughout. Accumulates in autophagic vacuoles due to a combination of ion trapping and specific interactions with membrane lipids. Has biological applications in measuring cardiac autophagic flux, studying Parkinson's disease, peritoneal ovarian tumor dissemination and respiratory diseases. This fluorescent tag is preferred over other due to its specificity and simplicity in detecting autophagic vesicles. Fluorescent substrate for assaying transamidating enzymes. Shown to inhibit endocytosis, the fibrin-stabilizing factor and epidermal growth factor (EGF) internalization. Dansyl dyes have environmentally sensitive fluorescence quantum yields and emission maxima along with large stokes shifts. Spectral Data: lambdaex 335nm; lambdaem518 nm in methanol.
MDL:
MFCD00042704
Molecular Formula:
C17H25N3O2S
Molecular Weight:
335.46
Package Type:
Vial
Product Description:
Fluorescent probe for derivatization of aldehydes, ketones and carboxylic acids. Used to prepare small fluorescent biomolecules via amidation or reductive amination. Used to monitor autophagy. Functions by marking the autophagosomes, which allows researchers to follow the molecular components of the process throughout. Accumulates in autophagic vacuoles due to a combination of ion trapping and specific interactions with membrane lipids. Has biological applications in measuring cardiac autophagic flux, studying Parkinson's disease, peritoneal ovarian tumor dissemination and respiratory diseases. This fluorescent tag is preferred over other due to its specificity and simplicity in detecting autophagic vesicles. Fluorescent substrate for assaying transamidating enzymes. Shown to inhibit endocytosis, the fibrin-stabilizing factor and epidermal growth factor (EGF) internalization. Dansyl dyes have environmentally sensitive fluorescence quantum yields and emission maxima along with large stokes shifts. Spectral Data: lambdaex 335nm; lambdaem518 nm in methanol.
Purity:
>98% (NMR)
SMILES:
CN(C)C1=C(C=CC=C2S(NCCCCCN)(=O)=O)C2=CC=C1
Solubility Chemicals:
Soluble in methanol or DMSO.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) L. Lorand, et al.; Anal. Biochem. 93, 453 (1979) | (2) H.T. Haigler, et al.; J. Biol. Chem. 255, 1239 (1980) | (3) R.R. Hantgan; Biochemistry 21, 1821 (1982) | (4) E. Perez-Paya, et al.; FEBS Lett. 278, 51 (1991) | (5) C.M. Gomez, et al.; Int. J. Biol. Macromol. 27, 291 (2000) | (6) D.B. Munafo & M.I. Colombo; J Cell Sci. 114, 3619 (2001) | (7) A. Campisi, et al.; Brain. Res. 978, 24 (2003) | (8) Y. Deguchi, et al.; Neurochem. 84, 1154 (2003) | (9) R.W. Sabnis; Handbook of biological dyes and stains (2010)