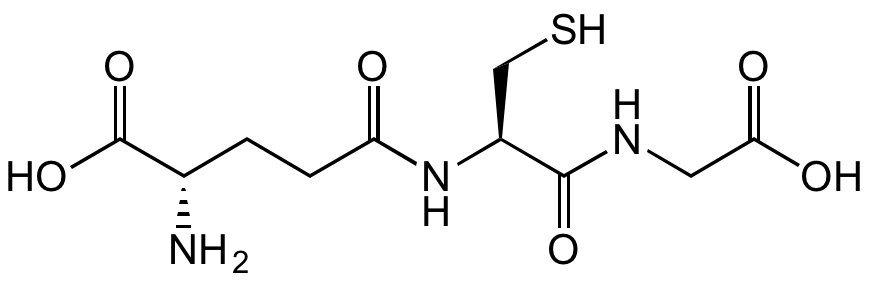
L-Glutathione reduced
| Code | Size | Price |
|---|
| CDX-G0005-G005 | 5 g | £53.00 |
Quantity:
| CDX-G0005-G025 | 25 g | £126.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short Term: +4°C. Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
GSH; L-gamma-glutamyl-L-cysteinyl-glycine
Appearance:
White crystalline powder.
CAS:
70-18-8
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C10H17N3O6S/c11-5(10(18)19)1-2-7(14)13-6(4-20)9(17)12-3-8(15)16/h5-6,20H,1-4,11H2,(H,12,17)(H,13,14)(H,15,16)(H,18,19)/t5-,6-/m0/s1
InChiKey:
RWSXRVCMGQZWBV-WDSKDSINSA-N
Long Description:
Chemical. CAS: 70-18-8. Formula: C10H17N3O6S. MW: 307.32. Synthetic. Major tripeptide widely distributed in both plants and animals. Endogenous antioxidant that plays a major role in reducing reactive oxygen species formed during cellular metabolism and the respiratory burst. Regulates activity of the redox sensitive transcription factor NF-kappaB. Cytoprotective. Serves as a nucleophilic co-substrate to glutathione transferases in the detoxification of xenobiotics and is an essential electron donor to glutathione peroxidases in the reduction of hydroperoxides. Involved in amino acid transport and maintenance of protein sulfhydryl reduction status. Posseses several metabolic, regulatory and protective functions. Forms disulfide bonds with cysteine residues in proteins.
MDL:
MFCD00065939
Molecular Formula:
C10H17N3O6S
Molecular Weight:
307.32
Package Type:
Vial
Product Description:
Major tripeptide widely distributed in both plants and animals. Endogenous antioxidant that plays a major role in reducing reactive oxygen species formed during cellular metabolism and the respiratory burst. Regulates activity of the redox sensitive transcription factor NF-kappaB. Cytoprotective. Serves as a nucleophilic co-substrate to glutathione transferases in the detoxification of xenobiotics and is an essential electron donor to glutathione peroxidases in the reduction of hydroperoxides. Involved in amino acid transport and maintenance of protein sulfhydryl reduction status. Posseses several metabolic, regulatory and protective functions. Forms disulfide bonds with cysteine residues in proteins.
Purity:
>98% (Titration)
SMILES:
OC([C@H](CCC(N[C@@H](CS)C(NCC(O)=O)=O)=O)N)=O
Solubility Chemicals:
Soluble in water (20 mg/ml) or PBS (10mg/ml). Sparingly soluble in DMSO or ethanol.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) A. Pompella, et al.; Biochem. Pharmacol. 66, 1499 (2003) | (2) A. Pastore, et al.; Clin. Chim. Acta. 333, 19 (2003) | (3) A. Chatterjee; Nutrients 5, 525 (2013) | (4) E.V. Kalinina, et al.; Biochemistry 79, 1562 (2014) | (5) K. Aquilano, et al.; Front. Pharmacol. 5, 196 (2014) | (6) P. Diaz-Vivancos, et al.; Free Radic. Biol. Med. 89, 1154 (2015) | (7) T. Homma & J. Fujii; Curr. Drug Metab. 16, 560 (2015)