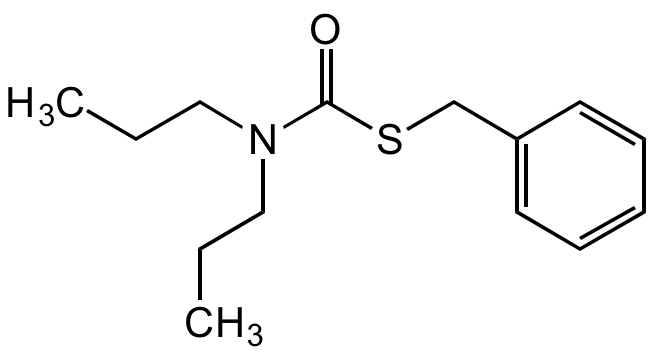
Prosulfocarb
| Code | Size | Price |
|---|
| CDX-P0187-M500 | 500 mg | £96.00 |
Quantity:
| CDX-P0187-G001 | 1 g | £157.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4 °C
Images
Documents
Further Information
Alternate Names/Synonyms:
S-Benzyl dipropylthiocarbamate; BRN 4804364
Appearance:
Buff or brown liquid.
CAS:
52888-80-9
Class:
9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS09
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H302, H317, H411
InChi:
InChI=1S/C14H21NOS/c1-3-10-15(11-4-2)14(16)17-12-13-8-6-5-7-9-13/h5-9H,3-4,10-12H2,1-2H3
InChiKey:
NQLVQOSNDJXLKG-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 52888-80-9. Formula: C14H21NOS. MW: 251.39. Synthetic. Thiocarbamate herbicide. Targets the meristematic region, or growing points, of plants, on both roots and shoots. Mode of action is through inhibition of lipid synthesis in plant cells. In particular, it inhibits fatty acid elongase enzymes, altering cell membranes and disrupting vital cell processes. It also effects the development of cutin and suberin, two waxy polymers that help to form plant cuticles. The result is that meristems are inhibited, root and shoot development is stunted, new leaves fail to emerge and shoots twist and turn dark green. Used for post-emergence control of grass and broad-leaved weeds in a wide range of crops. Compound can be used as analytical reference material.
MDL:
MFCD00145179
Molecular Formula:
C14H21NOS
Molecular Weight:
251.39
Package Type:
Vial
PG:
III
Precautions:
P273, P280
Product Description:
Thiocarbamate herbicide. Targets the meristematic region, or growing points, of plants, on both roots and shoots. Mode of action is through inhibition of lipid synthesis in plant cells. In particular, it inhibits fatty acid elongase enzymes, altering cell membranes and disrupting vital cell processes. It also effects the development of cutin and suberin, two waxy polymers that help to form plant cuticles. The result is that meristems are inhibited, root and shoot development is stunted, new leaves fail to emerge and shoots twist and turn dark green. Used for post-emergence control of grass and broad-leaved weeds in a wide range of crops. Compound can be used as analytical reference material.
Purity:
>97% (NMR)
Signal word:
Warning
SMILES:
O=C(SCC1=CC=CC=C1)N(CCC)CCC
Solubility Chemicals:
Soluble in chloroform, ethyl acetate, acetone, ethanol or methanol. Insoluble in water.
Source / Host:
Synthetic.
Transportation:
Excepted Quantity
UN Nummer:
UN3082
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) D.Callens & R. Bulcke; Meded. Fac. Landbouwwet, Univ. Gent 60, 141 (1995) | (2) The Pesticide Manual, 11th Edition: C. Tomlin; British Crop Protection Council (1997) | (3) M. Gennari, et al.; J. Environ. Sci. Health B 37, 297 (2002)
Related Products
| Product Name | Product Code | Supplier | Pyrithiobac | CDX-P0131 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyribenzoxim | CDX-P0132 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pyraflufen-ethyl | CDX-P0169 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quizalofop-p | CDX-Q0009 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||