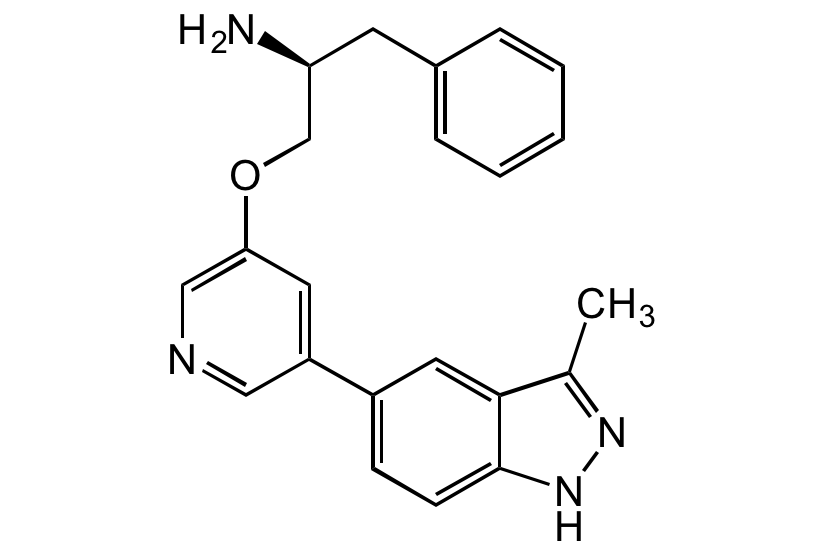
A-674563 (free base)
| Code | Size | Price |
|---|
| AG-CR1-3680-M001 | 1 mg | £50.00 |
Quantity:
| AG-CR1-3680-M005 | 5 mg | £130.00 |
Quantity:
| AG-CR1-3680-M010 | 10 mg | £200.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-200
Images
Documents
Further Information
Alternate Names/Synonyms:
alphaS-[[[5-(3-Methyl-1H-indazol-5-yl)-3-pyridinyl]oxy]methyl]-benzeneethanamine
Appearance:
Waxy solid.
CAS:
552325-73-2
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302, H332
InChi:
InChI=1S/C22H22N4O/c1-15-21-11-17(7-8-22(21)26-25-15)18-10-20(13-24-12-18)27-14-19(23)9-16-5-3-2-4-6-16/h2-8,10-13,19H,9,14,23H2,1H3,(H,25,26)/t19-/m0/s1
InChiKey:
BPNUQXPIQBZCMR-IBGZPJMESA-N
Long Description:
Chemical. CAS: 552325-73-2. Formula: C22H22N4O. MW: 358.4. Synthetic. A-674563 is a potent, ATP-competitive, reversible and orally available Akt1 inhibitor with an IC50 of 11nM. Binds to the ATP site of the AKT kinase domain, inhibiting AKT-catalyzed phosphorylation activity. Also inhibits PKA and CDK2 with IC50 values of 16nM and 46nM, respectively. 10- to >1,800-fold selective for Akt1 versus additional kinases in the CMGC, CAMK and TK families. Anticancer compound. Reduces phosphorylation of Akt downstream targets in cells and slows proliferation of tumor cells in vitro with an EC50 value of 0.4µM. Shown to decrease tumor growth in mouse tumor xenograft models. Decreased sphingosine kinase 1 (SphK1) activity in AML cells to deplete pro-survival sphingosine-1-phosphate (S1P) and boost pro-apoptotic ceramide production.
MDL:
MFCD11840461
Molecular Formula:
C22H22N4O
Molecular Weight:
358.4
Package Type:
Plastic Vial
Precautions:
P261, P271, P301, P312, P304, P340
Product Description:
A-674563 is a potent, ATP-competitive, reversible and orally available Akt1 inhibitor with an IC50 of 11nM. Binds to the ATP site of the AKT kinase domain, inhibiting AKT-catalyzed phosphorylation activity. Also inhibits PKA and CDK2 with IC50 values of 16nM and 46nM, respectively. 10- to >1,800-fold selective for Akt1 versus additional kinases in the CMGC, CAMK and TK families. Anticancer compound. Reduces phosphorylation of Akt downstream targets in cells and slows proliferation of tumor cells in vitro with an EC50 value of 0.4µM. Shown to decrease tumor growth in mouse tumor xenograft models. Decreased sphingosine kinase 1 (SphK1) activity in AML cells to deplete pro-survival sphingosine-1-phosphate (S1P) and boost pro-apoptotic ceramide production.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
CC1=NNC2=CC=C(C3=CC(OC[C@H](CC4=CC=CC=C4)N)=CN=C3)C=C21
Solubility Chemicals:
Soluble in DMF (20mg/ml), DMSO (10mg/ml) or ethanol (10mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo: Y. Luo, et al.; Mol. Cancer Ther. 4, 977 (2005) | Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity: N. Rhodes, et al.; Cancer Res. 68, 2366 (2008) | Soft tissue sarcoma cells are highly sensitive to AKT blockade: a role for p53-independent up-regulation of GADD45 alpha: Q.S. Zhu, et al.; Cancer Res. 68, 2895 (2008) | Enhanced sensitivity to sorafenib by inhibition of Akt1 expression in human renal cell carcinoma ACHN cells both in vitro and in vivo: H. Tei, et al.; Hum. Cell. 28, 114 (2015) | Unique roles of Akt1 and Akt2 in IGF-IR mediated lung tumorigenesis: S.E. Franks, et al.; Oncotarget 7, 3297 (2016) | Dual inhibition of AKT/FLT3-ITD by A674563 overcomes FLT3 ligand-induced drug resistance in FLT3-ITD positive AML: A. Wang, et al.; Oncotarget 7, 29131 (2016) | Concurrent targeting Akt and sphingosine kinase 1 by A-674563 in acute myeloid leukemia cells: L. Xu, et al.; BBRC 472, 662 (2016) | Pre-clinical assessment of A-674563 as an anti-melanoma agent: Y. Zou, et al.; BBRC 477, 1 (2016)