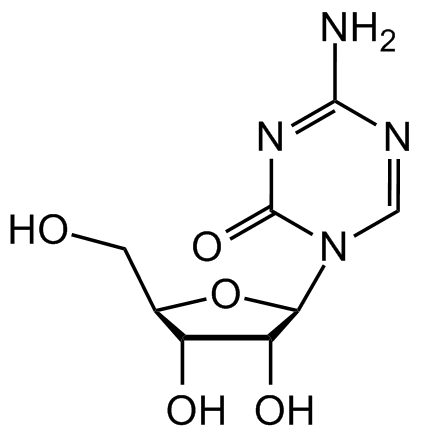
5-Azacytidine
| Code | Size | Price |
|---|
| CDX-A0271-M250 | 250 mg | £65.00 |
Quantity:
| CDX-A0271-G001 | 1 g | £188.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Images
Documents
Further Information
Alternate Names/Synonyms:
5-Azacitidine; Antibiotic U18496; 5-AzaC; Ladakamycin; Mylosar; NSC102816; NSC103-627; U18496; WR183027
Appearance:
White to off-white powder.
CAS:
320-67-2
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H302, H350
InChi:
InChI=1S/C8H12N4O5/c9-7-10-2-12(8(16)11-7)6-5(15)4(14)3(1-13)17-6/h2-6,13-15H,1H2,(H2,9,11,16)/t3-,4-,5-,6-/m1/s1
InChiKey:
NMUSYJAQQFHJEW-KVTDHHQDSA-N
Long Description:
Chemical. CAS: 320-67-2. Formula: C8H12N4O5. MW: 244.2. Synthetic. 5-Azacytidine, a chemical analogue of the DNA and RNA nucleoside cytidine, is an cell permeable inhibitor of DNA methyltransferases, potentially serving to reverse epigenetic changes. It reduces hypermethylation associated with certain diseases, including myelodysplastic syndromes (IC50s = 2.4 and 2.6µM for in vitro anti-myeloma activity) and cancer (IC50s ~ 0.4µM for inhibiting proliferation of various cancer cell lines). It has a reported half-life of 17 hours and is considerably cytotoxic; it must be incorporated into DNA to covalently trap DNA methyltransferases. Induces demethylation and reactivation of silenced genes. Improves the efficiency of reprogramming of stem cells; induces differentiation of mesenchymal stem cells into cardiomyocytes.
MDL:
MFCD00006539
Molecular Formula:
C8H12N4O5
Molecular Weight:
244.2
Package Type:
Vial
Precautions:
P201, P308+P313
Product Description:
5-Azacytidine, a chemical analogue of the DNA and RNA nucleoside cytidine, is an cell permeable inhibitor of DNA methyltransferases, potentially serving to reverse epigenetic changes. It reduces hypermethylation associated with certain diseases, including myelodysplastic syndromes (IC50s = 2.4 and 2.6µM for in vitro anti-myeloma activity) and cancer (IC50s ~ 0.4µM for inhibiting proliferation of various cancer cell lines). It has a reported half-life of 17 hours and is considerably cytotoxic; it must be incorporated into DNA to covalently trap DNA methyltransferases. Induces demethylation and reactivation of silenced genes. Improves the efficiency of reprogramming of stem cells; induces differentiation of mesenchymal stem cells into cardiomyocytes.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C=NC(N)=NC2=O)O1
Solubility Chemicals:
Soluble in ethanol, DMSO, DMF (30mg/ml), water or PBS (10mg/ml).
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) H. Kusaba, et al.; Eur. J. Biochem. 262, 924 (1999) | (2) B. Brueckner, et al.; Cancer Res. 65, 6305 (2005) | (3) F. Lyko & R. Brown; J. Natl. Cancer Inst. 97, 1498 (2005) | (4) C. Stresemann, et al.; Cancer Res. 66, 2794 (2006) | (5) M. Esteller; N. Engl. J. Med. 358, 1148 (2008) | (6) T.S. Mikkelsen, et al.; Nature 454, 49 (2008) | (7) A.M. Giraldo, et al.; Methods Mol. Biol. 791, 145 (2011) | (8) J.J. Chong, et al.; Cell Res. 22, 1932 (2013) | (9) M. Bhuvanagiri, et al.; EMBO Mol. Med. 6, 1593 (2014)
Related Products
| Product Name | Product Code | Supplier |
|---|