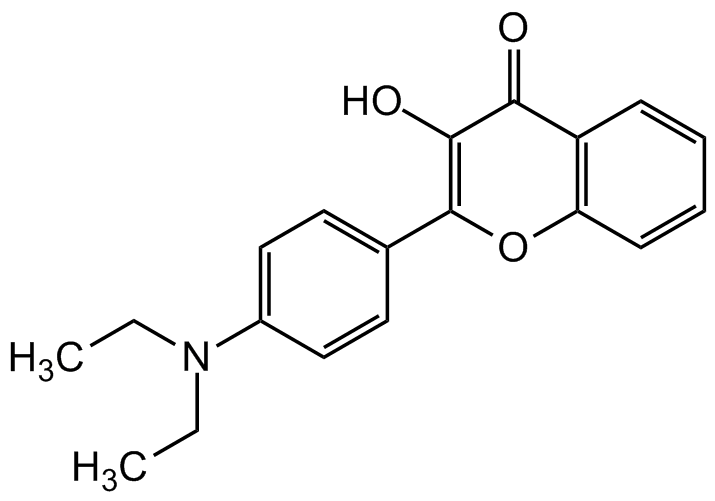
4'-Diethylamino-3-hydroxyflavone
| Code | Size | Price |
|---|
| CDX-D0202-M050 | 50 mg | £212.00 |
Quantity:
| CDX-D0202-M100 | 100 mg | £358.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Images
Documents
Further Information
Alternate Names/Synonyms:
DEAHF; FE; DHF; DMHF
Appearance:
Yellow powder.
CAS:
146680-78-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C19H19NO3/c1-3-20(4-2)14-11-9-13(10-12-14)19-18(22)17(21)15-7-5-6-8-16(15)23-19/h5-12,22H,3-4H2,1-2H3
InChiKey:
NTRKEYLXTPNPIY-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 146680-78-6. Formula: C19H19NO3. MW: 309.36. Synthetic. Fluorescent dye that exhibits dual band fluorescence emission which is sensitive to environmental factors. One band originates from the normal excited state (N*) and the other is due to the excited state intramolecular proton transfer (ESPIT) reaction product tautomer (T*). An increase in solvent polarity and hydrogen bonding ability of the solvent leads to an increase in the population of the N* form relative to the T* form. The intensity ratio of the normal and tautomeric fluorescence bands are near 510nm and 570nm, with a main absorption band (370-420nm). There exists a latent third emission band peaked at 535nm, which is reliably recorded upon excitation at wavelengths of 470-500nm. The positions of the two bands, as well as their intensity ratios are sensitive to the local environment, making many of these compounds attractive ratiometric fluorescent sensors. Attaching such fluorophores covalently to peptides and proteins, peptide-membrane interactions and structural changes of proteins can be studied. Furthermore, this dye may have important future applications as fluorescent sensors for the detection of adenosine triphosphate (ATP), as their excitation spectra are sensitive to ATP and this effect is selective over other nucleotides.
MDL:
MFCD30555921
Molecular Formula:
C19H19NO3
Molecular Weight:
309.36
Package Type:
Vial
Product Description:
Fluorescent dye that exhibits dual band fluorescence emission which is sensitive to environmental factors. One band originates from the normal excited state (N*) and the other is due to the excited state intramolecular proton transfer (ESPIT) reaction product tautomer (T*). An increase in solvent polarity and hydrogen bonding ability of the solvent leads to an increase in the population of the N* form relative to the T* form. The intensity ratio of the normal and tautomeric fluorescence bands are near 510nm and 570nm, with a main absorption band (370-420nm). There exists a latent third emission band peaked at 535nm, which is reliably recorded upon excitation at wavelengths of 470-500nm. The positions of the two bands, as well as their intensity ratios are sensitive to the local environment, making many of these compounds attractive ratiometric fluorescent sensors. Attaching such fluorophores covalently to peptides and proteins, peptide-membrane interactions and structural changes of proteins can be studied. Furthermore, this dye may have important future applications as fluorescent sensors for the detection of adenosine triphosphate (ATP), as their excitation spectra are sensitive to ATP and this effect is selective over other nucleotides.
Purity:
>90% (NMR)
SMILES:
O=C1C2=C(C=CC=C2)OC(C3=CC=C(N(CC)CC)C=C3)=C1O
Solubility Chemicals:
Soluble in DMSO, ethanol, methanol or acetonitrile.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) A. Sytnik, et al.; PNAS 91, 11968 (1994) | (2) Y.-M. Cheng, et al.; J. Phys. Chem. A 109, 11696 (2005) | (3) V.G. Pivorenko, et al.; J. Fluoresc. 16, 9 (2006) | (4) V.I. Tomin & R. Jaworski; Opt. Spectrosc. 109, 279 (2010) | (5) Y. Kimura, et al.; J. Phys. Chem. B 114, 11847 (2010) | (6) K. Hino, et al.; Bull. Chem. Soc. Jpn. 86, 721 (2013) | (7) D. Gosh, et al.; J. Phys. Chem. B 119, 5650 (2015) | (8) Z. Szakacs, et al.; Photochem. Photobiol. Sci. 15, 219 (2016) | (9) K. Furukawa, et al.; Chem. Phys. Lett. 643, 109 (2016)
Related Products
| Product Name | Product Code | Supplier | HPTS | CDX-H0034 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|