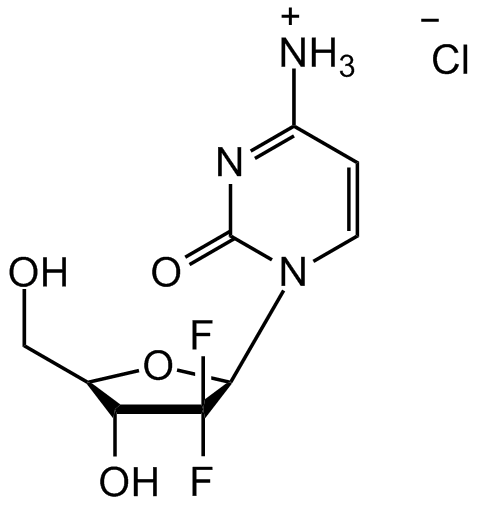
Gemcitabine hydrochloride
| Code | Size | Price |
|---|
| CDX-G0063-M010 | 10 mg | £53.00 |
Quantity:
| CDX-G0063-M050 | 50 mg | £157.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Images
Documents
Further Information
Alternate Names/Synonyms:
2'-Deoxy-2',2'-difluorocytidine; dFdC; Gemzar (Lilly); LY-188011; dFdC; dFdCyd; NSC613327
Appearance:
White to off-white powder.
CAS:
122111-03-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H360
InChi:
InChI=1S/C9H11F2N3O4.ClH/c10-9(11)6(16)4(3-15)18-7(9)14-2-1-5(12)13-8(14)17;/h1-2,4,6-7,15-16H,3H2,(H2,12,13,17);1H/t4-,6-,7-;/m1./s1
InChiKey:
OKKDEIYWILRZIA-OSZBKLCCSA-N
Long Description:
Chemical. CAS: 122111-03-9. Formula: C9H11F2N3O4 . HCl. MW: 263.20 . 36.46. Synthetic. Widely used antitumor agent in vitro and in vivo. Deoxycytidine analog with cytotoxic effects. Inhibits DNA synthesis and induces apoptosis through inhibition of ribonucleotide reductase (R1 and R2). Following the uptake of this prodrug into cells by nucleoside transporters it is phosphorylated to its mono(dFdCMP), di(dFdCDP), and triphosphorylated(dFdCTP) forms by deoxycytidine kinase. dFdCDP and dFdCTP are reported to inhibit the activity of ribonucleotide reductase and impede DNA synthesis and repair mechanisms and induce cell death. The triphosphate form is incorporated into DNA which induces masked chain termination and cell death. By specifically inhibiting growth arrest and DNA damage inducible protein 45 a (Gadd45a), a key mediator of active DNA demethylation, inhibits repair-mediated DNA demethylation in a methylation-sensitive reporter assay at concentrations ranging from 34-134nM. This anticancer nucleoside analog inhibits the growth of HL-60 promyelocytic leukemia cells with an LC50 value of 40nM. It inhibits the growth of MX-1 mammary, CX-1, HC-1, GC3, and VRC5 colon, LX-1, Calu-6 and NCI-H460 lung, and HS766T, PaCa-2, PANC-1, and BxPC-3 pancreatic cancer tumors in mouse xenograft models (45-93% inhibition). Also shown to block mitochondrial DNA polymerase gamma. Works synergistically with other chemotherapeutic agents to enhance their cytotoxicity. Has also broad antiretroviral activity, decreasing MuLV cell infectivity, a murine AIDS model, in cell culture (EC50 = ~1.5nM) and inhibits the progression of murine AIDS in vivo at a dose of 1-2 mg/kg per day.
MDL:
MFCD01735988
Molecular Formula:
C9H11F2N3O4 . HCl
Molecular Weight:
263.20 . 36.46
Package Type:
Vial
Precautions:
P201, P308+P313
Product Description:
Widely used antitumor agent in vitro and in vivo. Deoxycytidine analog with cytotoxic effects. Inhibits DNA synthesis and induces apoptosis through inhibition of ribonucleotide reductase (R1 and R2). Following the uptake of this prodrug into cells by nucleoside transporters it is phosphorylated to its mono(dFdCMP), di(dFdCDP), and triphosphorylated(dFdCTP) forms by deoxycytidine kinase. dFdCDP and dFdCTP are reported to inhibit the activity of ribonucleotide reductase and impede DNA synthesis and repair mechanisms and induce cell death. The triphosphate form is incorporated into DNA which induces masked chain termination and cell death. By specifically inhibiting growth arrest and DNA damage inducible protein 45 a (Gadd45a), a key mediator of active DNA demethylation, inhibits repair-mediated DNA demethylation in a methylation-sensitive reporter assay at concentrations ranging from 34-134nM. This anticancer nucleoside analog inhibits the growth of HL-60 promyelocytic leukemia cells with an LC50 value of 40nM. It inhibits the growth of MX-1 mammary, CX-1, HC-1, GC3, and VRC5 colon, LX-1, Calu-6 and NCI-H460 lung, and HS766T, PaCa-2, PANC-1, and BxPC-3 pancreatic cancer tumors in mouse xenograft models (45-93% inhibition). Also shown to block mitochondrial DNA polymerase gamma. Works synergistically with other chemotherapeutic agents to enhance their cytotoxicity. Has also broad antiretroviral activity, decreasing MuLV cell infectivity, a murine AIDS model, in cell culture (EC50 = ~1.5nM) and inhibits the progression of murine AIDS in vivo at a dose of 1-2 mg/kg per day.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
OC[C@@H]1[C@@H](O)C(F)(F)[C@H](N2C=CC([NH3+])=NC2=O)O1.[Cl-]
Solubility Chemicals:
Soluble in water (20mg/ml) or DMSO (slightly). Insoluble in ethanol.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) L.W. Hertel, et al.; Cancer Res. 50, 4417 (1990) | (2) V. Heinemann, et al.; Cancer Res. 52, 533 (1992) | (3) D.D. Ross & D.P. Cuddy; Biochem. Pharmacol. 48, 1619 (1994) | (5) W. Plunkett, et al.; Semin. Oncol. 22, 3 (1995) | (6) R.L. Merriman, et al.; New Drugs 14, 243 (1996) | (7) J.R. Mackey, et al.; Cancer Res. 58, 4349 (1998) | (8) R.P. McGeary, et al.; Mini Rev. Med. Chem. 8, 1384 (2008) | (9) C.L. Clouser, et al.; PLoS One 6, 1 (2011) | (10) S.W. Hung, et al.; Cancer Lett. 320, 138 (2012)
Related Products
| Product Name | Product Code | Supplier | 5-Fluorocytosine | CDX-F0077 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|