GSK2837808A
| Code | Size | Price |
|---|
| AG-CR1-3685-M001 | 1 mg | £50.00 |
Quantity:
| AG-CR1-3685-M005 | 5 mg | £170.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Target Species: Universal
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
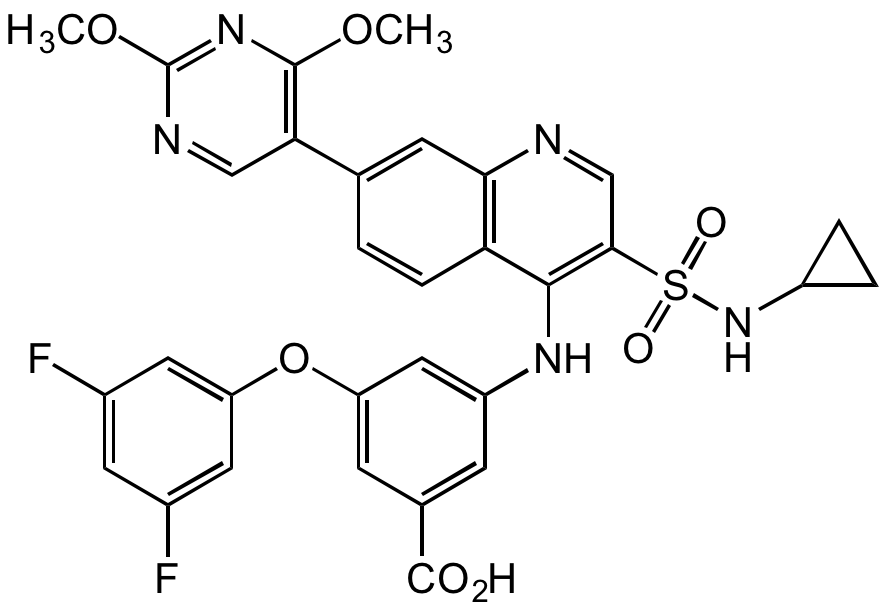
3-[[3-[(Cyclopropylamino)sulfonyl]-7-(2,4-dimethoxy-5-pyrimidinyl)-4-quinolinyl]amino]-5-(3,5-difluorophenoxy)benzoic acid
Appearance:
White to off-white solid.
CAS:
1445879-21-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H312, H317, H319, H335
InChi:
InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40)
InChiKey:
RZBCPMYJIARMGV-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1445879-21-9. Formula: C31H25F2N5O7S. MW: 649.6. . Potent and selective cell-permeable inhibitor of lactate dehydrogenase A (LDHA) (IC50 values are 2.6 and 43 nM for LDHA and LDHB, respectively). Decreases glycolysis. Useful agent for immunometabolism research. Exhibits antitumoral activity. Inhibits lactate production in selected cancer cell lines. Reduces glucose uptake and enhances mitochondrial oxygen consumption in Snu398 hepatocellular carcinoma cells. Inhibits proliferation and induces apoptosis in Snu398 cells. Inhibits transcription of histone 2B (H2B) gene in HCT116 and NCM460 cells. Lactate dehydrogenase A (LDHA) is an important enzyme involved in fermentative glycolysis, important in tumor initiation and metabolism. Cancer cells rely on increased glycolysis resulting in increased lactate production in addition to aerobic respiration in the mitochondria, even under normal oxygen concentrations (a process known as the Warburg effect). This renders LDHA a promising molecular target for the treatment of various cancers.
MDL:
MFCD28133406
Molecular Formula:
C31H25F2N5O7S
Molecular Weight:
649.6
Package Type:
Vial
Precautions:
P302+P352, P304+P340, P305+P351+P338
Product Description:
Potent and selective cell permeable inhibitor of lactate dehydrogenase A (LDHA) (IC50 values are 2.6 and 43 nM for LDHA and LDHB, respectively). Decreases glycolysis. Useful agent for immunometabolism research. Exhibits antitumoral activity. Inhibits lactate production in selected cancer cell lines. Reduces glucose uptake and enhances mitochondrial oxygen consumption in Snu398 hepatocellular carcinoma cells. Inhibits proliferation and induces apoptosis in Snu398 cells. Inhibits transcription of histone 2B (H2B) gene in HCT116 and NCM460 cells. Lactate dehydrogenase A (LDHA) is an important enzyme involved in fermentative glycolysis, important in tumor initiation and metabolism. Cancer cells rely on increased glycolysis resulting in increased lactate production in addition to aerobic respiration in the mitochondria, even under normal oxygen concentrations (a process known as the Warburg effect). This renders LDHA a promising molecular target for the treatment of various cancers.
Purity:
>98%
Signal word:
Warning
SMILES:
FC1=CC(F)=CC(OC2=CC(C(O)=O)=CC(NC3=C(C=NC4=C3C=CC(C5=C(N=C(N=C5)OC)OC)=C4)S(NC6CC6)(=O)=O)=C2)=C1
Solubility Chemicals:
Soluble in DMSO (30mg/ml) or DMF (30mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells: J. Billiard, et al.; Cancer Metab. 1, 19 (2013) | Targeting lactate dehydrogenase-A inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor initiating cells: H. Xie, et al.; Cell Metab. 19, 795 (2014) | A guide to immunometabolism for immunologists: L.A. O'Neill, et al.; Nat. Rev. Immunol. 16, 553 (2016) | The inhibition of lactate dehydrogenase A hinders the transcription of histone 2B gene independently from the block of aerobic glycolysis: E. Brighenti, et al.; BBRC 485, 742 (2017)
Related Products
| Product Name | Product Code | Supplier | Radicicol | AG-CN2-0021 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-Deoxy-D-glucose | AG-CR1-3681 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||