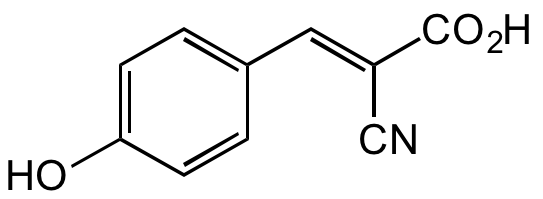
alpha-Cyano-4-hydroxycinnamic acid
| Code | Size | Price |
|---|
| AG-CR1-3686-G001 | 1 g | £50.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Target Species: Universal
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
alpha-Cyano-4-hydroxycinnamate; alpha-CCA; 4-HCCA; CHCA; NSC173138
Appearance:
White to yellow solid.
CAS:
28166-41-8
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light.
Hazards:
H317, H319
InChi:
InChI=1S/C10H7NO3/c11-6-8(10(13)14)5-7-1-3-9(12)4-2-7/h1-5,12H,(H,13,14)/b8-5+
InChiKey:
AFVLVVWMAFSXCK-VMPITWQZSA-N
Long Description:
Chemical. CAS: 28166-41-8. Formula: C10H7NO3. MW: 189.2. . Specific monocarboxylate transporter (MCT) inhibitor. Inhibits mitochondrial lactate and pyruvate transport. Decreases glycolysis. Useful agent for immunometabolism research. Exhibits antitumoral and antiangiogenic activity in gliomas in vivo; decreases glycolytic metabolism, migration and invasion. Blocks lactate efflux from glioma cells and sensitizes cells to irradiation. Highly malignant tumors rely heavily on aerobic glycolysis (metabolism of glucose to lactic acid even under presence of oxygen; Warburg Effect) and thus need to efflux lactic acid via MCTs to the tumor micro-environment to maintain a robust glycolytic flux and to prevent the tumor from apoptosis. Inhibiting lactic acid efflux is a very effective therapeutic strategy against highly glycolytic malignant tumors. Common organic matrix-assisted laser desorption/ionization (MALDI) matrix.
MDL:
MFCD00004204
Molecular Formula:
C10H7NO3
Molecular Weight:
189.2
Package Type:
Vial
Precautions:
P302+P352, P305+P351+P338
Product Description:
Specific monocarboxylate transporter (MCT) inhibitor. Inhibits mitochondrial lactate and pyruvate transport. Decreases glycolysis. Useful agent for immunometabolism research. Exhibits antitumoral and antiangiogenic activity in gliomas in vivo; decreases glycolytic metabolism, migration and invasion. Blocks lactate efflux from glioma cells and sensitizes cells to irradiation. Highly malignant tumors rely heavily on aerobic glycolysis (metabolism of glucose to lactic acid even under presence of oxygen; Warburg Effect) and thus need to efflux lactic acid via MCTs to the tumor micro-environment to maintain a robust glycolytic flux and to prevent the tumor from apoptosis. Inhibiting lactic acid efflux is a very effective therapeutic strategy against highly glycolytic malignant tumors. Common organic matrix-assisted laser desorption/ionization (MALDI) matrix.
Purity:
>98%
Signal Word:
Warning
SMILES:
OC1=CC=C(/C=C(C#N)/C(O)=O)C=C1
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or methanol (5mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle: G.A. Brooks, et al.; PNAS 96, 1129 (1999) | Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: effect of monocarboxylate transporter inhibition: A.R. Diers, et al.; Biochem. J. 444, 561 (2012) | The monocarboxylate transporter inhibitor alpha-cyano-4-hydroxycinnamic acid disrupts rat lung branching: S. Granja, et al.; Cell Physiol. Biochem. 32, 1845 (2013) | A guide to immunometabolism for immunologists: L.A. O'Neill, et al.; Nat. Rev. Immunol. 16, 553 (2016) | Similarities and Distinctions of Cancer and Immune Metabolism in Inflammation and Tumors: G. Andrejeva & J.C. Rathmell; Cell Metab. 26, 49 (2017) | Metabolic Instruction of Immunity: M.D. Buck, et al.; Cell 169, 570 (2017)