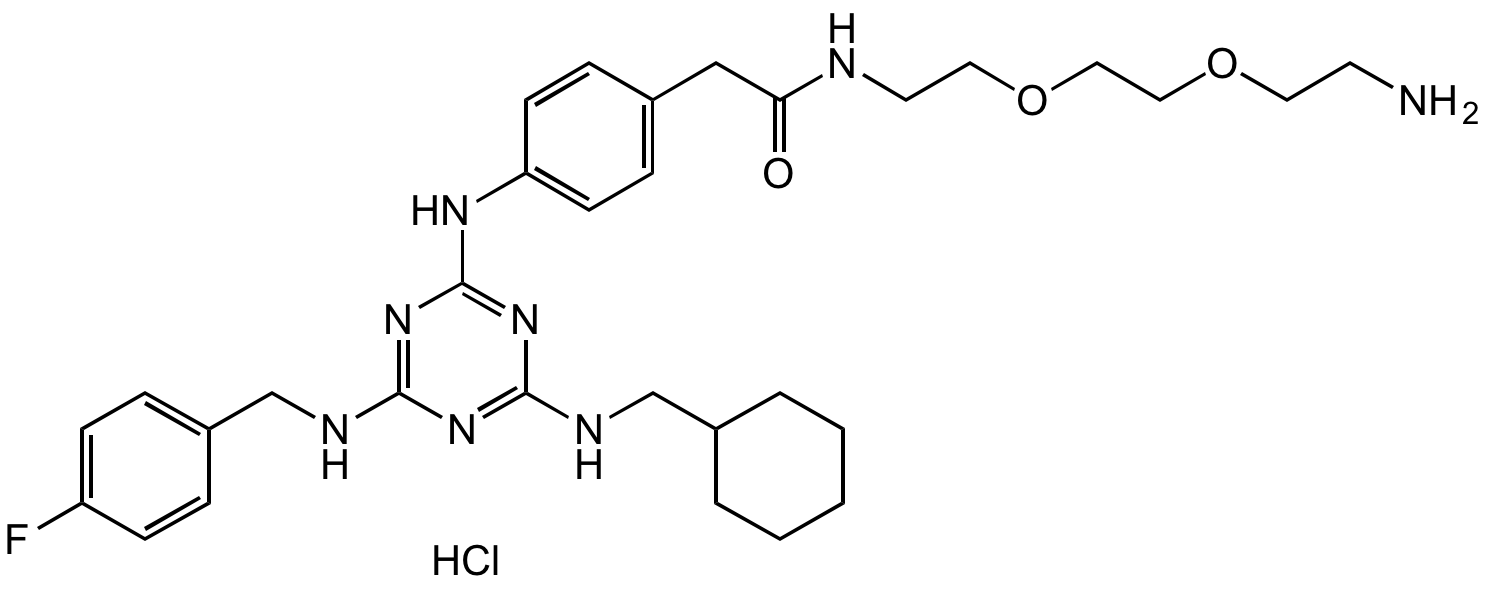
AP-III-a4 hydrochloride
| Code | Size | Price |
|---|
| AG-CR1-3696-M001 | 1 mg | £75.00 |
Quantity:
| AG-CR1-3696-M005 | 5 mg | £270.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Target Species: Universal
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
ENOblock
Appearance:
White to off-white solid.
CAS:
1177827-73-4 (free base)
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302
InChi:
InChI=1S/C31H43FN8O3.ClH/c32-26-10-6-25(7-11-26)22-36-30-38-29(35-21-24-4-2-1-3-5-24)39-31(40-30)37-27-12-8-23(9-13-27)20-28(41)34-15-17-43-19-18-42-16-14-33;/h6-13,24H,1-5,14-22,33H2,(H,34,41)(H3,35,36,37,38,39,40);1H
InChiKey:
UYGRNXLHKIBHMM-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1177827-73-4 (free base). Formula: C31H43FN8O3 . HCl. MW: 594.7 . 36.5. . Cell-permeable first non-substrate analog enolase inhibitor (IC50=0.576µM). A recent study suggested no directly blocking of enolase activity in vitro and a indirect mechanism. Useful agent for immunometabolism research. The glycolysis enzymes enolase is known to have additional, non-glycolytic roles in cellular physiology, which has been termed 'moonlighting'. Anticancer agent. Inhibits cancer cell metastasis in a zebrafish cancer cell xenograft model. Induces cell death under hypoxia and inhibits cancer cell migration and invasion by down-regulation of AKT and Bcl-xL expression. Antidiabetic agent. Reduced hyperglycemia and hyperlipidemia in mice. Reduced blood glucose, LDL cholesterol and enolase activity in T2DM mice. Had beneficial effects on lipid homeostasis, fibrosis, inflammatory markers, nephrotoxicity and cardiac hypertrophy. Down-regulates phosphoenolpyruvate carboxykinase and sterol regulatory element-binding protein-1, which are known to produce anti-diabetic effects. Induced glucose uptake and inhibited phosphoenolpyruvate carboxykinase (PEPCK) expression in vitro. Reduced neuron specific enolase (NSE) levels and suppressed neuroinflammation in an acute spinal cord injury (SCI) model.
MDL:
MFCD28167782
Molecular Formula:
C31H43FN8O3 . HCl
Molecular Weight:
594.7 . 36.5
Package Type:
Vial
Precautions:
P301+P312
Product Description:
Cell permeable first non-substrate analog enolase inhibitor (IC50=0.576µM). A recent study suggested no direct blocking of enolase activity in vitro and a indirect mechanism. Useful agent for immunometabolism research. The glycolysis enzymes enolase is known to have additional, non-glycolytic roles in cellular physiology, which has been termed 'moonlighting'. Anticancer agent. Inhibits cancer cell metastasis in a zebrafish cancer cell xenograft model. Induces cell death under hypoxia and inhibits cancer cell migration and invasion by down-regulation of AKT and Bcl-xL expression. Antidiabetic agent. Reduced hyperglycemia and hyperlipidemia in mice. Reduced blood glucose, LDL cholesterol and enolase activity in T2DM mice. Had beneficial effects on lipid homeostasis, fibrosis, inflammatory markers, nephrotoxicity and cardiac hypertrophy. Down-regulates phosphoenolpyruvate carboxykinase and sterol regulatory element-binding protein-1, which are known to produce anti-diabetic effects. Induced glucose uptake and inhibited phosphoenolpyruvate carboxykinase (PEPCK) expression in vitro. Reduced neuron-specific enolase (NSE) levels and suppressed neuroinflammation in an acute spinal cord injury (SCI) model.
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
FC(C=C1)=CC=C1CNC2=NC(NC3=CC=C(CC(NCCOCCOCCN)=O)C=C3)=NC(NCC4CCCCC4)=N2.Cl
Solubility Chemicals:
Soluble in DMSO (20mg/ml), ethanol (20mg/ml) or water (20mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
A unique small molecule inhibitor of enolase clarifies its role in fundamental biological processes: D.W. Jung, et al.; ACS Chem. Biol. 8, 1271 (2013) | ENOblock Does Not Inhibit the Activity of the Glycolytic Enzyme Enolase: N. Satani, et al.; PLoS One 11, e0168739 (2016) | ENOblock, a unique small molecule inhibitor of the non-glycolytic functions of enolase, alleviates the symptoms of type 2 diabetes: H. Cho, et al.; Sci. Rep. 7, 44186 (2017) | Targeting Enolase in Reducing Secondary Damage in Acute Spinal Cord Injury in Rats: A. Haque, et al.; Neurochem. Res. 42, 2777 (2017)