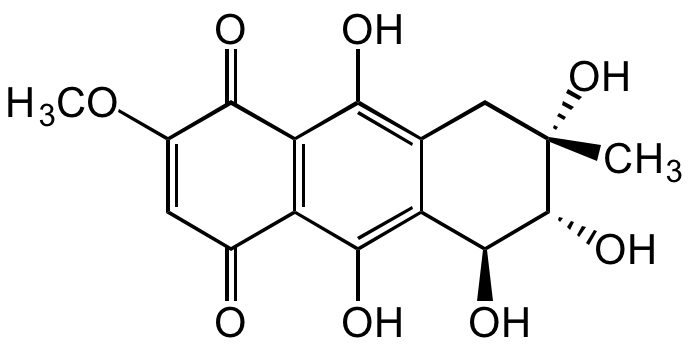
Bostrycin
Product Code: AG-CN2-0175
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0175-C250 | 250 ug | £90.00 |
Quantity:
| AG-CN2-0175-M001 | 1 mg | £230.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Rhodosporin; 5,6,7,8-Tetrahydro-5,6,7,9,10-pentahydroxy-2-methoxy-7-methyl-1,4-anthracenedione
Appearance:
Dark red solid.
CAS:
21879-81-2
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C16H16O8/c1-16(23)4-5-8(14(21)15(16)22)13(20)9-6(17)3-7(24-2)12(19)10(9)11(5)18/h3,14-15,18,20-23H,4H2,1-2H3/t14-,15+,16-/m0/s1
InChiKey:
ZQNOLGRKZRDRQO-XHSDSOJGSA-N
Long Description:
Chemical. CAS: 21879-81-2. Formula: C16H16O8. MW: 336.3. Isolated from fungus Arthrinium sp. Anthraquinone antibiotic. Red antibacterial agent against Gram positive bacteria. Anticancer agent. Inhibits cell proliferation of human lung carcinoma A549 cells via downregulation of the PI3K/Akt pathway. Also shown to block cell cycle at G1 phase leading to cell death. Induced mitochondria-mediated apoptosis in the yeast Saccharomyces cerevisiae. Weak in vitro inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase (MptpB). Potential red-colored agent for food processing.
MDL:
MFCD01664500
Molecular Formula:
C16H16O8
Molecular Weight:
336.3
Package Type:
Vial
Product Description:
Anthraquinone antibiotic. Red antibacterial agent against Gram positive bacteria. Anticancer agent. Inhibits cell proliferation of human lung carcinoma A549 cells via downregulation of the PI3K/Akt pathway. Also shown to block cell cycle at G1 phase leading to cell death. Induced mitochondria-mediated apoptosis in the yeast Saccharomyces cerevisiae. Weak in vitro inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase (MptpB). Potential red-colored agent for food processing.
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
O=C(C(OC)=C1)C2=C(C(O)=C([C@H](O)[C@@H](O)[C@](O)(C)C3)C3=C2O)C1=O
Solubility Chemicals:
Soluble in DMSO (1mg/ml).
Source / Host:
Isolated from fungus Arthrinium sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Structure of bostrycin: T. Noda, et al.; Tetrahed. Lett. 9, 6087 (1968) | The structure of bostrycin: T. Noda, et al.; Tetrahedron 26, 1339 (1970) | The antibiotic bostrycin from Alternaria Eichhorniae: K.L. Stevens, et al.; Phytochem. 18, 1579 (1979) | Bostrycin: S.Y. Chen, et al.; Acta Crystallogr. Sect. E Struct. Rep. Online 64, o2226 (2008) | The anthracenedione compound bostrycin induces mitochondria-mediated apoptosis in the yeast Saccharomyces cerevisiae: C. Xu, et al.; FEMS Yeast Res. 10, 297 (2010) | Bostrycin inhibits proliferation of human lung carcinoma A549 cells via downregulation of the PI3K/Akt pathway: W.S. Chen, et al.; J. Exp. Clin. Cancer Res. 30, 17 (2011) | Bostrycin, a novel coupling agent for protein immobilization and prevention of biomaterial-centered infection produced by Nigrospora sp. No. 407: W.J. Yang, et al.; Enzyme Microb. Technol. 50, 287 (2012) | Studies on the synthesis of derivatives of marine-derived bostrycin and their structure-activity relationship against tumor cells: H. Chen, et al.; Mar. Drugs 10, 932 (2012) | Identification of Bostrycin Derivatives as Potential Inhibitors of Mycobacterium tuberculosis Protein Tyrosine Phosphatase (MptpB): D.N. Chen, et al.; Med. Chem. 12, 296 (2016) | Bostrycin production by agro-industrial residues and its potential for food processing: Y.-H. Huang, et al.; Food Sci. Biotech. 26, 715 (2017)