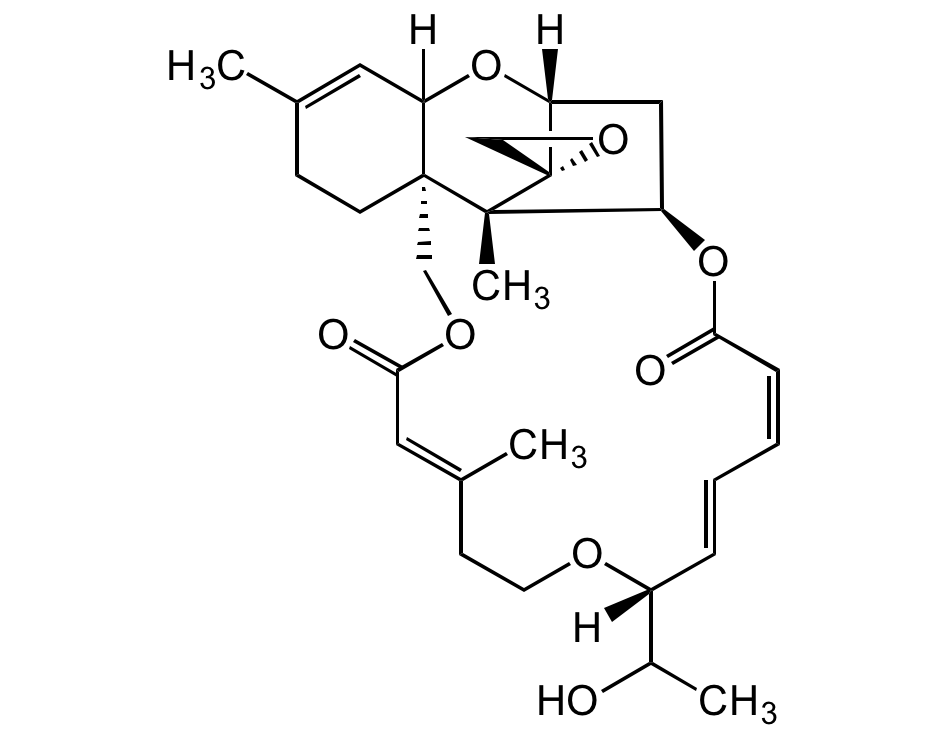
Roridin E
Product Code: AG-CN2-0176
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0176-C250 | 250 ug | £65.00 |
Quantity:
| AG-CN2-0176-M001 | 1 mg | £175.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Satratoxin D; 2',3'-Didehydro-7'-deoxo-2'-deoxy-7'-(1-hydroxy-ethyl)verrucarin A
Appearance:
Off-white solid.
CAS:
16891-85-3
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302
InChi:
InChI=1S/C29H38O8/c1-18-9-11-28-16-34-26(32)14-19(2)10-12-33-21(20(3)30)7-5-6-8-25(31)37-22-15-24(36-23(28)13-18)29(17-35-29)27(22,28)4/h5-8,13-14,20-24,30H,9-12,15-17H2,1-4H3/b7-5+,8-6-,19-14+/t20?,21-,22-,23?,24-,27-,28-,29+/m1/s1
InChiKey:
KEEQQEKLEZRLDS-ALFAKORJSA-N
Long Description:
Chemical. CAS: 16891-85-3. Formula: C29H38O5. MW: 514.6. Isolated from fungus Trichoderma sp. Mycotoxin. Implicated in human and animal toxicosis. Potent cytotoxic and antiproliferative agent against cancer cell lines. Potent antimalarial agent. Antifungal, antibiotic, phototoxic and cytostatic agent. Antiviral against arenavirus Junin (JUNV).
MDL:
MFCD01675256
Molecular Formula:
C29H38O5
Molecular Weight:
514.6
Package Type:
Vial
Precautions:
P301+P312
Product Description:
Mycotoxin. Implicated in human and animal toxicosis. Potent cytotoxic and antiproliferative agent against cancer cell lines. Potent antimalarial agent. Antifungal, antibiotic, phototoxic and cytostatic agent. Antiviral against arenavirus Junin (JUNV).
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
[H]C12[C@@]3([C@]4(C)[C@]5(CO5)[C@](C[C@H]4OC(/C=CC=C[C@](OCC/C(C)=C/C(OC3)=O)([H])C(O)C)=O)([H])O2)CCC(C)=C1
Solubility Chemicals:
Soluble in ethanol, methanol or DMSO (all 1mg/ml).
Source / Host:
Isolated from fungus Trichoderma sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Structure of the antibiotic Roridin E: P. Traxler, et al.; Helv. Chim. Acta 53, 2071 (1970) | Effects of macrocyclic trichothecene mycotoxins on the murine immune system: B.J. Hughes, et al.; Arch. Environ. Contam. Toxicol. 18, 388 (1989) | Antimalarial activity of macrocyclic trichothecenes isolated from the fungus Myrothecium verrucaria: M. Isaka, et al.; J. Nat. Prod. 62, 329 (1999) | Phytotoxicity and mammalian cytotoxicity of macrocyclic trichothecene mycotoxins from Myrothecium verrucaria: H.K. Abbas, et al.; Phytochem. 59, 309 (2002) | Evaluation of the antiviral activity against Junin virus of macrocyclic trichothecenes produced by the hypocrealean epibiont of Baccharis coridifolia: C.C. Garcia, et al.; Planta Med. 68, 209 (2002) | 12'-Hydroxyl group remarkably reduces Roridin E cytotoxicity: T. Oda, et al.; Mycosciences 51, 317 (2010) | Isolation and characterization of roridin E: C.D. Ridge, et al.; MRC 55, 337 (2017) | Preparative separation and purification of trichothecene mycotoxins from the marine fungus Fusarium sp. LS68 by high-speed countercurrent chromatography in stepwise elution mode: Y. Liu, et al.; Mar. Drugs 16, 73 (2018)
Related Products
| Product Name | Product Code | Supplier |
|---|