GE81112 A/B
Product Code: AG-CN2-0306
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0306-M001 | 1 mg | £90.00 |
Quantity:
| AG-CN2-0306-M005 | 5 mg | £300.00 |
Quantity:
| AG-CN2-0306-M025 | 25 mg | £880.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Antibiotic GE81112A/Antibiotic GE81112B Mixture
Appearance:
White to off-white powder.
CAS:
883726-13-4 [GE81112A]883726-14-5 [GE81112B]
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.Protect from light when in solution.
InChi:
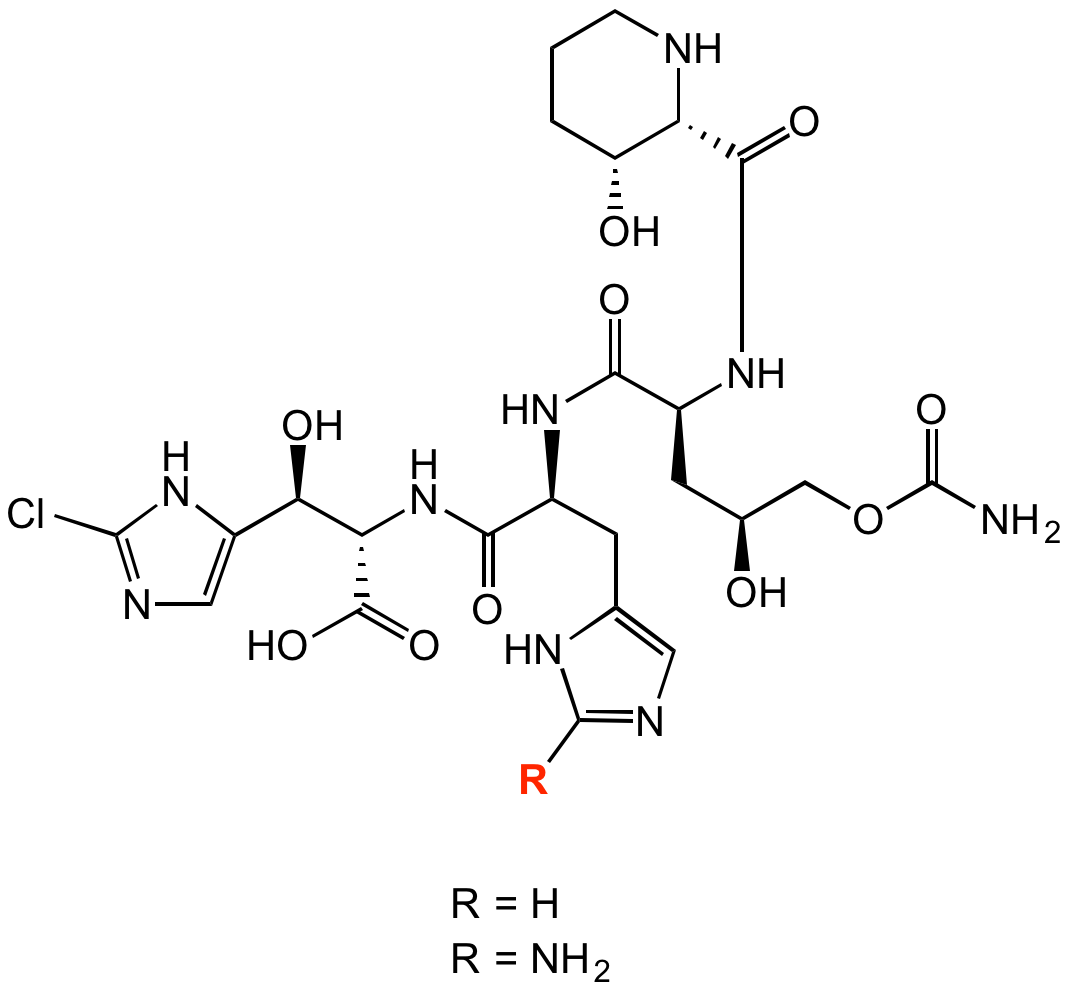
R=H: InChI=1S/C24H34ClN9O10/c25-23-29-7-14(33-23)18(37)17(22(41)42)34-20(39)12(4-10-6-27-9-30-10)31-19(38)13(5-11(35)8-44-24(26)43)32-21(40)16-15(36)2-1-3-28-16/h6-7,9,11-13,15-18,28,35-37H,1-5,8H2,(H2,26,43)(H,27,30)(H,29,33)(H,31,38)(H,32,40)(H,34,39)(H,41,42)/t11-,12-,13-,15+,16-,17-,18+/m0/s1
R=NH2: InChI=1S/C24H35ClN10O10/c25-22-29-7-13(34-22)17(38)16(21(42)43)35-19(40)11(4-9-6-30-23(26)31-9)32-18(39)12(5-10(36)8-45-24(27)44)33-20(41)15-14(37)2-1-3-28-15/h6-7,10-12,14-17,28,36-38H,1-5,8H2,(H2,27,44)(H,29,34)(H,32,39)(H,33,41)(H,35,40)(H,42,43)(H3,26,30,31)/t10-,11-,12-,14+,15-,16-,17+/m0/s1
InChiKey:
R=H: MPRVLYUMDIXCGD-XYYQMEIKSA-N
R=NH2: UFNFLYFKIYFJJJ-WJAIJCGZSA-N
Long Description:
Chemical. CAS: 883726-13-4 [GE81112A], 883726-14-5 [GE81112B]. Formula: C24H34ClN9O10 [A], C24H35ClN10O10 [B]. MW: 644.0 [A]659.1 [B]. Isolated from Strepomyces sp. Tetrapeptide antibiotic. Potent and selective inhibitor of bacterial protein synthesis. Translational inhibitor specific for the initiation phase. Binds to the 30S ribosomal subunit and specifically inhibits P-site decoding of the mRNA initiation codon by the fMet-tRNA anticodon. Inhibits in vivo protein synthesis but not other cell functions such as DNA duplication, transcription and cell wall synthesis. Antibacterial activity against some Gram-positive and Gram-negative bacteria. Unique scaffold for designing new drugs.
Molecular Formula:
C24H34ClN9O10 [A]
C24H35ClN10O10 [B]
Molecular Weight:
644.0 [A]659.1 [B]
Other data:
Purity Note:The purity is referred exclusively to the main congener(s), the sample also contains minor related congeners. See: Novel tetrapeptide inhibitors of bacterial protein synthesis produced by a Streptomyces sp: L. Brandi, et al.; Biochemistry 45, 3692 (2006)
Package Type:
Vial
Product Description:
Tetrapeptide antibiotic. Potent and selective inhibitor of bacterial protein synthesis. Translational inhibitor specific for the initiation phase. Binds to the 30S ribosomal subunit and specifically inhibits P-site decoding of the mRNA initiation codon by the fMet-tRNA anticodon. Inhibits in vivo protein synthesis but not other cell functions such as DNA duplication, transcription and cell wall synthesis. Antibacterial activity against some Gram-positive and Gram-negative bacteria. Unique scaffold for designing new drugs.
Purity:
>90% (HPLC, for A/B)
SMILES:
R=H: O[C@H](C1=CN=C(Cl)N1)[C@@H](C(O)=O)NC([C@@H](NC([C@H](C[C@H](O)COC(N)=O)NC([C@@H]2[C@@H](CCCN2)O)=O)=O)CC3=CN=C([H])N3)=O
R=NH2: O[C@H](C1=CN=C(Cl)N1)[C@@H](C(O)=O)NC([C@@H](NC([C@H](C[C@H](O)COC(N)=O)NC([C@@H]2[C@@H](CCCN2)O)=O)=O)CC3=CN=C(N)N3)=O
Solubility Chemicals:
Soluble in DMSO or water.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Specific, efficient, and selective inhibition of prokaryotic translation initiation by a novel peptide antibiotic: L. Brandi, et al.; PNAS 103, 39 (2006) | Novel tetrapeptide inhibitors of bacterial protein synthesis produced by a Streptomyces sp: L. Brandi, et al.; Biochemistry 45, 3692 (2006) | Inhibition of translation initiation complex formation by GE81112 unravels a 16S rRNA structural switch involved in P-site decoding: A. Fabbretti, et al.; PNAS 113, E2286 (2016) | The Oligopeptide Permease Opp Mediates Illicit Transport of the Bacterial P-site Decoding Inhibitor GE81112: A. Maio, et al.; Antibiotics 24, 5 (2016) | Structure of a 30S pre-initiation complex stalled by GE81112 reveals structural parallels in bacterial and eukaryotic protein synthesis initiation pathways: J.P. Lopez-Alonso, et al.; Nucleic Acids Res. 45, 2179 (2017) | Total synthesis and structural revision of the antibiotic tetrapeptide GE81112A: G. Jurjens, et al.; Angew. Chem. Int. Ed. 57, 20157(2018)
Related Products
| Product Name | Product Code | Supplier | Actagardin | AG-CN2-0300 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GE2270A | AG-CN2-0303 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GE2270 D2 | AG-CN2-0304 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GE23077 A1/B1 | AG-CN2-0305 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NAI-107 [Microbisporicin] | AG-CN2-0307 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NAI-112 | AG-CN2-0309 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||