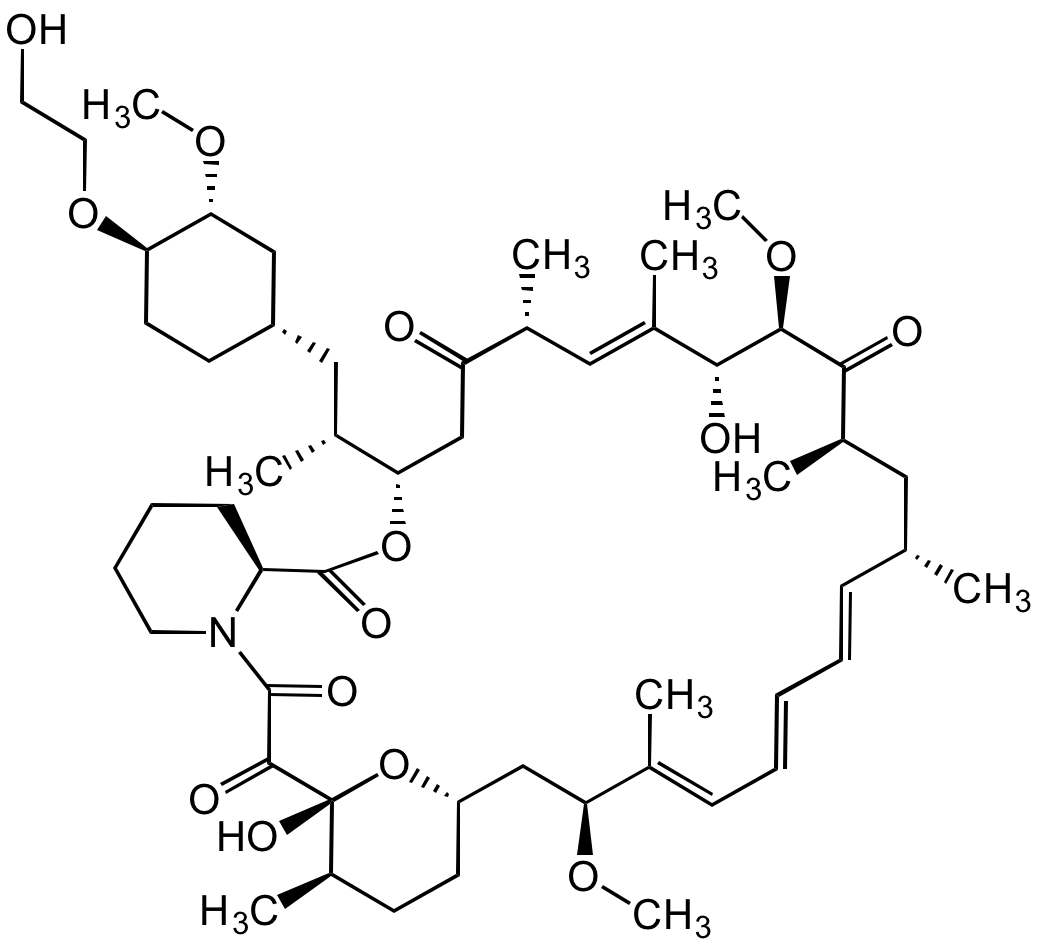
Everolimus
Product Code: AG-CN2-0520
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0520-M001 | 1 mg | £40.00 |
Quantity:
| AG-CN2-0520-M005 | 5 mg | £65.00 |
Quantity:
| AG-CN2-0520-M025 | 25 mg | £160.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
42-O-(2-Hydroxyethyl)rapamycin; NVP-RAD001; RAD001; SDZRAD; Zortress; Afinitor; Certican; Votubia
Appearance:
White to off-white solid.
CAS:
159351-69-6
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H361, H372
InChi:
InChI=1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1
InChiKey:
HKVAMNSJSFKALM-GKUWKFKPSA-N
Long Description:
Chemical. CAS: 159351-69-6. Formula: C53H83NO14. MW: 958.2. Isolated from Streptomyces hygroscopicus. Macrolide antibiotic, inhibiting bacterial protein synthesis. Orally available Rapamycin derivative that shows improved pharmacokinetics and pharmacodynamics. Potent immunosuppressant. Binds with high affinity to the FK506 binding protein-12 (FKBP-12) to generate an immunosuppressive complex that inhibits the activation of the mammalian target of rapamycin (mTOR). More selective for the mTORC1 protein complex, with little impact on the mTORC2 complex, compared to Rapamycin. Since mTORC2 is believed to play an important role in glucose metabolism and the immune system, selective inhibition of mTORC1 achieves many of the benefits of rapamycin without the associated glucose intolerance and immunosuppression Anticancer agent. Inhibition of mTOR reduces the activity of effectors downstream, which leads to a blockage in the progression of cells from G1 into S phase, and subsequently inducing cell growth arrest, apoptosis and autophagy, resulting in reduction of cell proliferation, angiogenesis and glucose uptake. Inhibits tumor proliferation in vitro and in vivo. Currently used as an immunosuppressant to prevent rejection of organ transplants and in the treatment of renal cell cancer and other tumours.
MDL:
MFCD07785165
Molecular Formula:
C53H83NO14
Molecular Weight:
958.2
Package Type:
Vial
Precautions:
P308+P313
Product Description:
Macrolide antibiotic, inhibiting bacterial protein synthesis. Orally available rapamycin derivative that shows improved pharmacokinetics and pharmacodynamics. Potent immunosuppressant. Binds with high affinity to the FK506 binding protein-12 (FKBP12) to generate an immunosuppressive complex that inhibits the activation of the mammalian target of rapamycin (mTOR). More selective for the mTORC1 protein complex, with little impact on the mTORC2 complex, compared to rapamycin. Since mTORC2 is believed to play an important role in glucose metabolism and the immune system, selective inhibition of mTORC1 achieves many of the benefits of rapamycin without the associated glucose intolerance and immunosuppression Anticancer agent. Inhibition of mTOR reduces the activity of effectors downstream, which leads to a blockage in the progression of cells from G1 into S phase, and subsequently inducing cell growth arrest, apoptosis and autophagy, resulting in reduction of cell proliferation, angiogenesis and glucose uptake. Inhibits tumor proliferation in vitro and in vivo. Used as an immunosuppressant to prevent rejection of organ transplants and in the treatment of renal cell cancer and other tumors.
Purity:
>97% (HPLC)
Signal word:
Danger
SMILES:
OCCO[C@@H]1CC[C@@H](C[C@H]([C@@H]2CC([C@@H](/C=C([C@H]([C@H](C([C@@H](C[C@@H](/C=C/C=C/C=C([C@H](C[C@@H]3CC[C@H]([C@@](O3)(C(C(N4CCCC[C@H]4C(O2)=O)=O)=O)O)C)OC)C)C)C)=O)OC)O)C)C)=O)C)C[C@H]1OC
Solubility Chemicals:
Soluble in DMSO (50mg/ml), ethanol (50mg/ml), methanol or DMF. Insoluble in water.
Source / Host:
Isolated from Streptomyces hygroscopicus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
SDZ RAD, a new rapamycin derivative: synergism with cyclosporine: H.J. Schuurman, et al.; Transplantation 64, 32 (1997) | SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo: W. Schuler, et al.; Transplantation 64, 36 (1997) | mTOR inhibitors: an overview: P. Neuhaus, et al.; Liver Transpl. 7, 473 (2001) (Review) | Review of the proliferation inhibitor everolimus: B. Nashan; Expert Opin. Investig. Drugs. 11, 1845 (2002) (Review) | The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation: I. Beuvink, et al.; Cell 120, 747 (2005) | Everolimus: an immunosuppressive agent in transplantation: J.K. Patel & J.A. Kobashigawa; Expert Opin. Pharmacother. 7, 1347 (2006) (Review) | Recent developments in targeting the mammalian target of rapamycin (mTOR) kinase pathway: P. Smolewski; Anticancer Drugs 17, 487 (2006) (Review) | The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells: K. Zitzmann, et al.; Neuroendocrinology 85, 54 (2007) | Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML: Z. Zeng, et al.; Blood 109, 3509 (2007) | Inhibition of mTOR pathway by everolimus cooperates with EGFR inhibitors in human tumours sensitive and resistant to anti-EGFR drugs: R. Bianco, et al.; Br. J. Cancer 98, 923 (2008) | Everolimus: an update on the mechanism of action, pharmacokinetics and recent clinical trials: A.I. Sanchez-Fructuoso; Expert Opin. Drug Metab. Toxicol. 4, 807 (2008) (Review) | mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor: H.A. Lane, et al.; Clin. Cancer Res. 15, 1612 (2009) | Everolimus - a new approach in the treatment of renal cell carcinoma: G. Anandappa, et al.; Cancer Manag. Res. 2, 61 (2010) | Development of everolimus, a novel oral mTOR inhibitor, across a spectrum of diseases: D. Lebwohl, et al.; Ann. N. Y. Acad. Sci. 1291, 14 (2013) (Review) | Cellular and molecular effects of the mTOR inhibitor everolimus: U. Saran, et al.; Clin. Sci. 129, 895 (2015) (Review) | Everolimus is a new anti-cancer molecule: Metabolic side effects as lipid disorders and hyperglycemia: L. Morviducci, et al.; Diabetes Res. Clin. Pract. 143, 428 (2018) (Review)
Related Products
| Product Name | Product Code | Supplier | FK-506 | AG-CN2-0047 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|