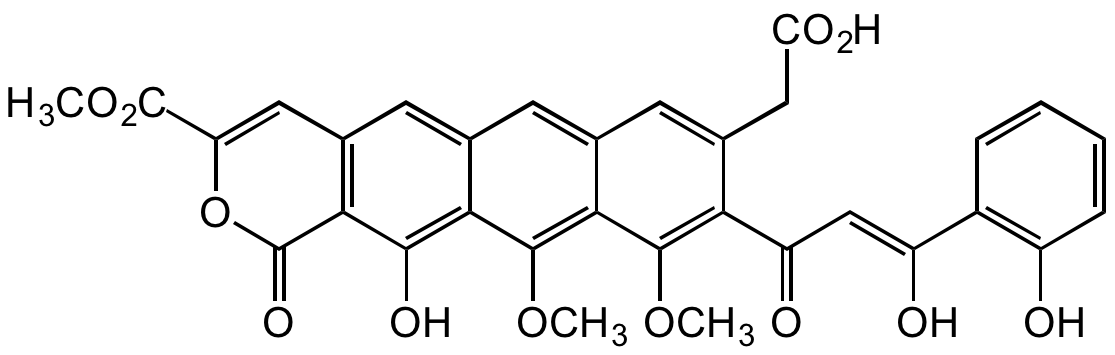
Thermorubin
Product Code: AG-CN2-0339
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0339-M001 | 1 mg | £90.00 |
Quantity:
| AG-CN2-0339-M005 | 5 mg | £300.00 |
Quantity:
| AG-CN2-0339-M025 | 25 mg | £880.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Thermorubin A; NSC105760
Appearance:
Red to violet powder.
CAS:
37577-75-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.Protect from light when in solution.
InChi:
InChI=1S/C32H24O12/c1-41-29-24(21(35)13-20(34)18-6-4-5-7-19(18)33)17(12-23(36)37)10-15-8-14-9-16-11-22(31(39)43-3)44-32(40)26(16)28(38)25(14)30(42-2)27(15)29/h4-11,13,33-34,38H,12H2,1-3H3,(H,36,37)/b20-13-
InChiKey:
GGEDVBUCHDZLTH-MOSHPQCFSA-N
Long Description:
Chemical. CAS: 37577-75-6. Formula: C32H24O12. MW: 600.5. Isolated from Thermoactinomyces sp. Anthracene antibiotic. Antibacterial agent. Active against both Gram-positive and Gram-negative bacteria. Inhibits the initiation stage of bacterial protein synthesis by binding to the inter-subunit bridge B2a on the 70S ribosome. Does not inhibit DNA and RNA synthesis. Potent aldose reductase inhibitor.
Molecular Formula:
C32H24O12
Molecular Weight:
600.5
Package Type:
Vial
Product Description:
Anthracene antibiotic. Antibacterial agent. Active against both Gram-positive and Gram-negative bacteria. Inhibits the initiation stage of bacterial protein synthesis by binding to the inter-subunit bridge B2a on the 70S ribosome. Does not inhibit DNA and RNA synthesis. Potent aldose reductase inhibitor.
Purity:
>85% (HPLC)
SMILES:
O=C1OC(C(OC)=O)=CC2=C1C(O)=C(C(OC)=C(C(OC)=C(C(/C=C(O)/C3=C(O)C=CC=C3)=O)C(CC(O)=O)=C4)C4=C5)C5=C2
Solubility Chemicals:
Soluble in DMSO, aqueous acetonitrile or ethanol.
Source / Host:
Isolated from Thermoactinomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Structure of thermorubin A, the major orange-red antibiotic of Thermoactinomyces antibioticus: C.E. Moppett, et al.; JACS 94, 3269 (1972) | The protein synthesis inhibitor thermorubin. 1. Nature of the thermorubin-ribosome complex: F. Lin & A. Wishnia; Biochem. 21, 477 (1982) | The protein synthesis inhibitor thermorubin. 2. Mechanism of inhibition of initiation on Escherichia coli ribosomes: F. Lin & A. Wishnia; Biochem. 21, 484 (1982) | Synthesis and antibacterial activity of some derivatives of the antibiotic thermorubin: B. Cavalleri, et al.; J. Antibiot. 38, 1752 (1985) | Thermorubin and 2-hydroxyphenyl acetic acid, aldose reductase inhibitors: K. Hayashi, et al.; J. Antibiot. 48, 1345 (1995) | The antibiotic thermorubin inhibits protein synthesis by binding to inter-subunit bridge B2a of the ribosome: D. Bulkley, et al.; J. Mol. Biol. 416, 571 (2012)
Related Products
| Product Name | Product Code | Supplier | NAI-802 | AG-CN2-0310 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAI-857 | AG-CN2-0311 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NAI-97 [Planosporicin] | AG-CN2-0312 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ramoplanin A2 | AG-CN2-0318 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||