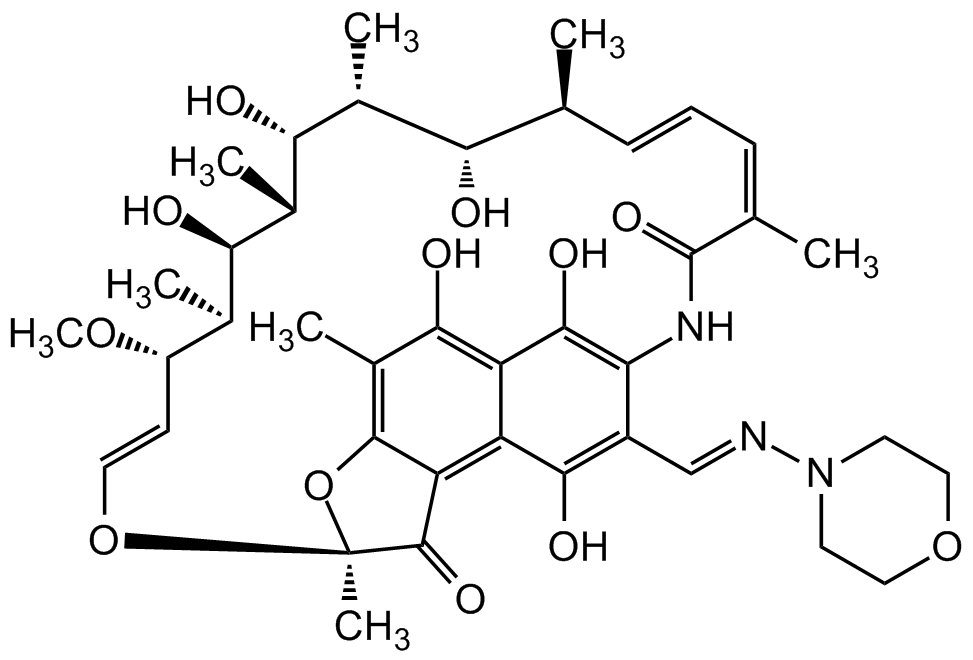
Rifamycin AMI-DA
Product Code:
AG-CN2-0330
AG-CN2-0330
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CN2-0330-M010 | 10 mg | £120.00 |
Quantity:
| AG-CN2-0330-M050 | 50 mg | £450.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Rifamycin AMI/DA; NCI 143-463 ; NSC 143463; 3-(Morpholin-4-ylimino-methyl)-O25-deacetyl-rifamycin
Appearance:
Red powder.
CAS:
17096-02-5
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.Protect from light when in solution.
InChi:
InChI=1S/C40H53N3O12/c1-19-10-9-11-20(2)39(51)42-30-25(18-41-43-13-16-53-17-14-43)35(48)27-28(36(30)49)34(47)24(6)37-29(27)38(50)40(7,55-37)54-15-12-26(52-8)21(3)32(45)23(5)33(46)22(4)31(19)44/h9-12,15,18-19,21-23,26,31-33,44-49H,13-14,16-17H2,1-8H3,(H,42,51)/b10-9+,15-12+,20-11-,41-18+/t19-,21+,22+,23-,26?,31-,32+,33+,40-/m0/s1
InChiKey:
DVSYDJZNVVAOFS-ITMIFSLQSA-N
Long Description:
Chemical. CAS: 17096-02-5. Formula: C40H53N3O12. MW: 767.9. Semisynthetic. Ansamycin antibiotic. Selective inhibitor of bacterial DNA-dependent RNA polymerase (RNAP). Effective against mycobacteria, and are therefore used in research of tuberculosis, leprosy and mycobacterium avium complex (MAC) infections.
Molecular Formula:
C40H53N3O12
Molecular Weight:
767.9
Package Type:
Vial
Product Description:
Ansamycin antibiotic. Selective inhibitor of bacterial DNA-dependent RNA polymerase (RNAP). Effective against mycobacteria and therefore used in research of tuberculosis, leprosy and Mycobacterium avium complex (MAC) infections.
Purity:
>90% (HPLC)
SMILES:
OC1=C(NC(/C(C)=CC=C[C@H](C)[C@H](O)[C@@H](C)[C@H]([C@@H](C)[C@@H]([C@H](C)[C@H](/C=C/O2)OC)O)O)=O)C(/C=N/N3CCOCC3)=C(O)C4=C5C(O[C@@]2(C)C5=O)=C(C)C(O)=C41
Solubility Chemicals:
Soluble in DMSO, aqueous acetonitrile or ethanol.
Source / Host:
Semisynthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Desacetyl-rifamycins: preparation and antibacterial properties: N. Maggi, et al.; Experientia 24, 209 (1968) | Rifamycin antibiotics: inhibitors of Rauscher murine leukemia virus reverse transcriptase and of purified DNA polymerases from human normal and leukemic lymphoblasts: S.S. Yang, et al.; J. Natl. Cancer Inst. 49, 7 (1972) | Rifamycin Derivatives Strongly Inhibiting RNA>DNA Polymerase (Reverse Transcriptase) of Murine Sarcoma Viruses: C. Gurgo, et al.; J. Natl. Cancer Inst. 49, 61 (1972)
Related Products
| Product Name | Product Code | Supplier | Rifamycin AF | AG-CN2-0321 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rifamycin AF-API | AG-CN2-0325 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin AF-EPTAPI | AG-CN2-0326 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin AF-DA | AG-CN2-0328 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin AG | AG-CN2-0329 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin AMP-DA | AG-CN2-0331 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin M14 | AG-CN2-0332 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin O | AG-CN2-0333 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin PR-14 | AG-CN2-0334 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin PR-3 | AG-CN2-0335 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin S, 8-Methyl- | AG-CN2-0337 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||