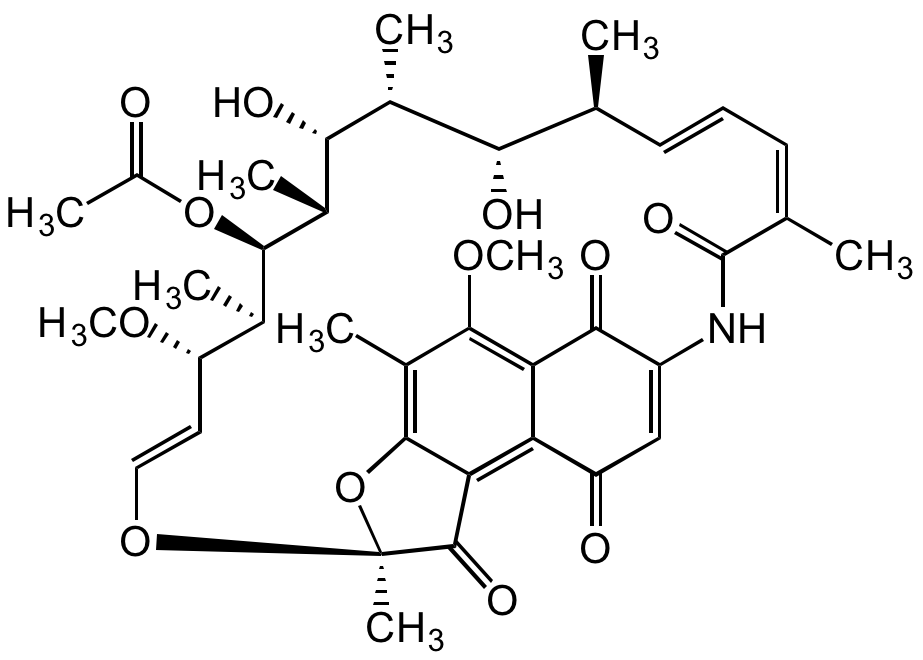
Rifamycin S, 8-Methyl-
Product Code: AG-CN2-0337
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0337-M010 | 10 mg | £120.00 |
Quantity:
| AG-CN2-0337-M050 | 50 mg | £450.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
8-Methylrifamycin S; 8-O-Methylrifamycin S; 1,4-Dideoxy-1,4-dihydro-8-O-methyl-1,4-dioxo-rifamycin
Appearance:
Yellow to orange powder.
CAS:
42898-13-5
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.Protect from light when in solution.
InChi:
InChI=1S/C38H47NO12/c1-17-12-11-13-18(2)37(46)39-24-16-25(41)27-28(32(24)44)34(48-10)22(6)35-29(27)36(45)38(8,51-35)49-15-14-26(47-9)19(3)33(50-23(7)40)21(5)31(43)20(4)30(17)42/h11-17,19-21,26,30-31,33,42-43H,1-10H3,(H,39,46)/b12-11+,15-14+,18-13-/t17-,19+,20+,21+,26?,30-,31+,33+,38-/m0/s1
InChiKey:
CWRGYNMOWZQIMN-KSWODUNLSA-N
Long Description:
Chemical. CAS: 42898-13-5. Formula: C38H47NO12. MW: 709.8. Semisynthetic. Ansamycin antibiotic. Selective inhibitor of bacterial DNA-dependent RNA polymerase (RNAP). Effective against mycobacteria, and are therefore used in research of tuberculosis, leprosy and mycobacterium avium complex (MAC) infections.
Molecular Formula:
C38H47NO12
Molecular Weight:
709.8
Package Type:
Vial
Product Description:
Ansamycin antibiotic. Selective inhibitor of bacterial DNA-dependent RNA polymerase (RNAP). Effective against mycobacteria and therefore used in research of tuberculosis, leprosy and Mycobacterium avium complex (MAC) infections.
Purity:
>90% (HPLC)
SMILES:
O=C1C(NC(/C(C)=CC=C[C@H](C)[C@H](O)[C@@H](C)[C@H]([C@@H](C)[C@@H]([C@H](C)[C@H](/C=C/O2)OC)OC(C)=O)O)=O)=CC(C3=C4C(O[C@@]2(C)C4=O)=C(C)C(OC)=C31)=O
Solubility Chemicals:
Soluble in DMSO, aqueous acetonitrile or ethanol.
Source / Host:
Semisynthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
The constitution and configuration of rifamycins B, O, S and SV: W. Oppolzer & V. Prelog; Helv. Chim. Acta 56, 2287 (1973) | Letter: Electronegative groups at C-3 of rifamycin S enhance its activity toward DNA-dependent RNA polymerase: M.F. Dampier & H.W. Whitlock; JACS 97, 6254 (1975)
Related Products
| Product Name | Product Code | Supplier | Rifamycin AF | AG-CN2-0321 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rifamycin AF-API | AG-CN2-0325 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin AF-EPTAPI | AG-CN2-0326 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin AF-DA | AG-CN2-0328 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin AG | AG-CN2-0329 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin AMI-DA | AG-CN2-0330 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin AMP-DA | AG-CN2-0331 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin M14 | AG-CN2-0332 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin O | AG-CN2-0333 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin PR-14 | AG-CN2-0334 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rifamycin PR-3 | AG-CN2-0335 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||