Liraglutide
| Code | Size | Price |
|---|
| AG-CP3-0034-M001 | 1 mg | £80.00 |
Quantity:
| AG-CP3-0034-M005 | 5 mg | £170.00 |
Quantity:
| AG-CP3-0034-M025 | 25 mg | £400.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Appearance:
White to off-white powder.
CAS:
204656-20-2
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C172H265N43O51/c1-18-20-21-22-23-24-25-26-27-28-29-30-37-53-129(224)195-116(170(265)266)59-64-128(223)180-68-41-40-50-111(153(248)199-115(62-67-135(232)233)154(249)204-120(73-100-44-33-31-34-45-100)159(254)214-140(93(11)19-2)167(262)192-97(15)146(241)201-122(76-103-79-183-108-49-39-38-48-106(103)108)157(252)203-118(72-90(5)6)158(253)212-138(91(7)8)165(260)200-110(52-43-70-182-172(177)178)149(244)184-81-130(225)193-109(51-42-69-181-171(175)176)148(243)187-84-137(236)237)196-144(239)95(13)189-143(238)94(12)191-152(247)114(58-63-127(174)222)194-131(226)82-185-151(246)113(61-66-134(230)231)198-155(250)117(71-89(3)4)202-156(251)119(75-102-54-56-105(221)57-55-102)205-162(257)124(85-216)208-164(259)126(87-218)209-166(261)139(92(9)10)213-161(256)123(78-136(234)235)206-163(258)125(86-217)210-169(264)142(99(17)220)215-160(255)121(74-101-46-35-32-36-47-101)207-168(263)141(98(16)219)211-132(227)83-186-150(245)112(60-65-133(228)229)197-145(240)96(14)190-147(242)107(173)77-104-80-179-88-188-104/h31-36,38-39,44-49,54-57,79-80,88-99,107,109-126,138-142,183,216-221H,18-30,37,40-43,50-53,58-78,81-87,173H2,1-17H3,(H2,174,222)(H,179,188)(H,180,223)(H,184,244)(H,185,246)(H,186,245)(H,187,243)(H,189,238)(H,190,242)(H,191,247)(H,192,262)(H,193,225)(H,194,226)(H,195,224)(H,196,239)(H,197,240)(H,198,250)(H,199,248)(H,200,260)(H,201,241)(H,202,251)(H,203,252)(H,204,249)(H,205,257)(H,206,258)(H,207,263)(H,208,259)(H,209,261)(H,210,264)(H,211,227)(H,212,253)(H,213,256)(H,214,254)(H,215,255)(H,228,229)(H,230,231)(H,232,233)(H,234,235)(H,236,237)(H,265,266)(H4,175,176,181)(H4,177,178,182)/t93-,94-,95-,96-,97-,98+,99+,107-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,138-,139-,140-,141-,142-/m0/s1
InChiKey:
YSDQQAXHVYUZIW-QCIJIYAXSA-N
Long Description:
Chemical. CAS: 204656-20-2. Formula: C172H265N43O51. MW: 3751.2. Synthetic. Long-acting acylated glucagon-like peptide-1 (GLP-1) receptor agonist. Antidiabetic and antiobesity agent used clinically to treat type 2 diabetes mellitus. Binding to GLP-1R, activates AMP-activated protein kinase, consequently stimulates insulin secretion in pancreatic beta cells and suppresses glucagon secretion in a glucose-dependent manner. Improves control of blood glucose and consequently modulates appetite and body weight. Inhibits beta cell apoptosis and improves beta cell mass. Shown to ameliorate glycometabolism and insulin resistance through the upregulation of GLUT4. Neuroprotective. Prevents neurodegenerative processes in pathologies such as Alzheimer's and Parkinsons Disease. Anti-inflammatory agent. Exerts cardioprotective roles via activating prosurvival pathways and suppressing inflammation. Anti-pyroptotic by inhibiting TNF-alpha and hypoxia-induced inflammasome activation. Anticancer agent. Inhibited proliferation and induced apoptosis in cancer cell lines. Shown to inhibit osteoclastogenesis.
MDL:
MFCD28126980
Molecular Formula:
C172H265N43O51
Molecular Weight:
3751.2
Package Type:
Vial
Product Description:
Long-acting acylated glucagon-like peptide-1 (GLP-1) receptor agonist. Antidiabetic and antiobesity agent used clinically to treat type 2 diabetes mellitus. Binding to GLP-1R, activates AMP-activated protein kinase, consequently stimulates insulin secretion in pancreatic beta cells and suppresses glucagon secretion in a glucose-dependent manner. Improves control of blood glucose and consequently modulates appetite and body weight. Inhibits beta cell apoptosis and improves beta cell mass. Shown to ameliorate glycometabolism and insulin resistance through the upregulation of GLUT4. Neuroprotective. Prevents neurodegenerative processes in pathologies such as Alzheimer's and Parkinsons Disease. Anti-inflammatory agent. Exerts cardioprotective roles via activating prosurvival pathways and suppressing inflammation. Anti-pyroptotic by inhibiting TNF-alpha and hypoxia-induced inflammasome activation. Anticancer agent. Inhibited proliferation and induced apoptosis in cancer cell lines. Shown to inhibit osteoclastogenesis.
Purity:
>98% (HPLC)
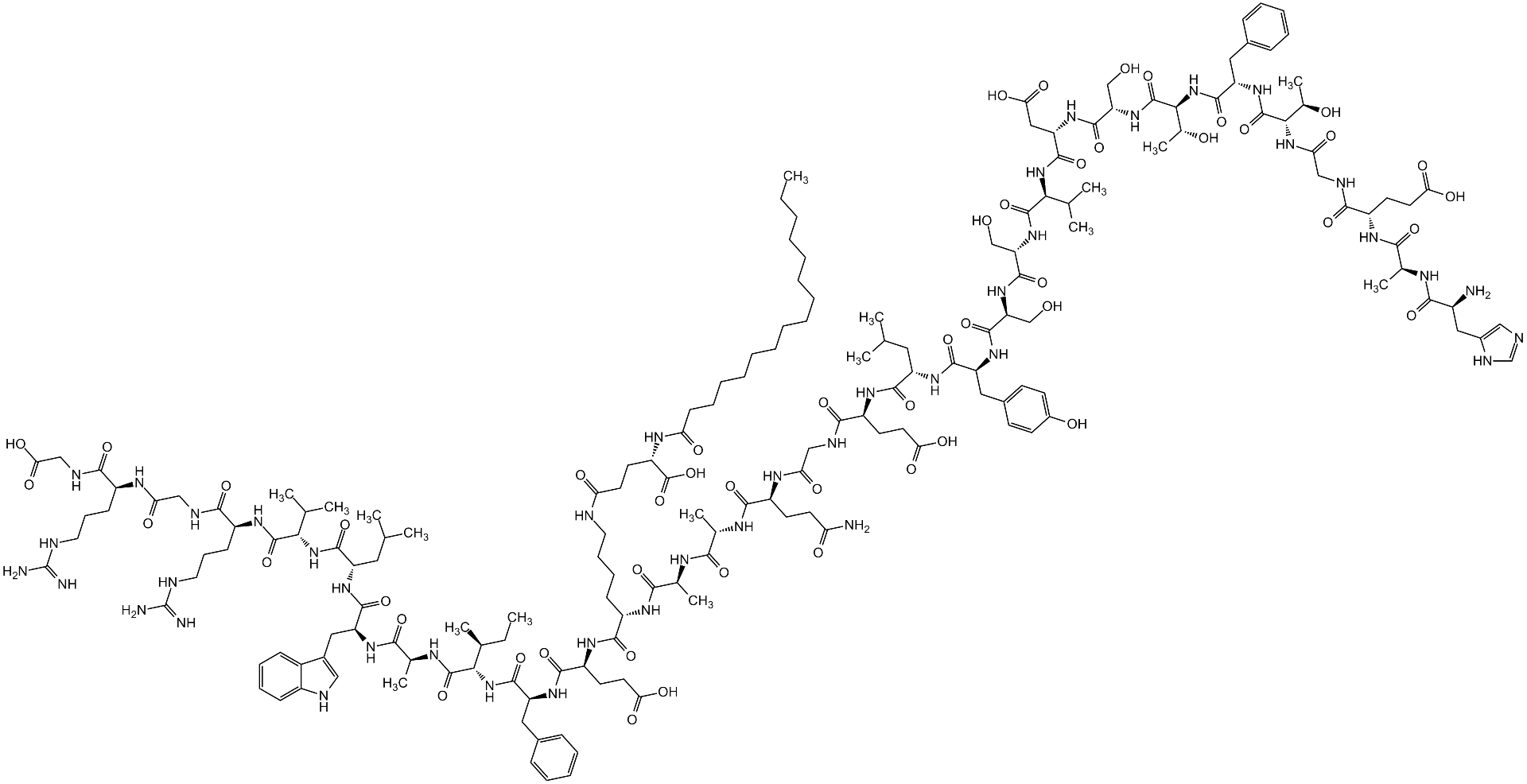
Sequence:
H-His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys(|A-Glu-palmitoyl)-Glu-Phe-Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH
SMILES:
O=C([C@H](CC(C)C)N([H])C([C@H](CC1=CN([H])C2=CC=CC=C12)N([H])C([C@H](C)N([H])C([C@H]([C@@H](C)CC)N([H])C([C@H](CC3=CC=CC=C3)N([H])C([C@H](CCC(O)=O)N([H])C([C@H](CCCCN([H])C(CC[C@@H](C(O)=O)N([H])C(CCCCCCCCCCCCCCC)=O)=O)N([H])C([C@H](C)N([H])C([C@H](C)N([H])C([C@H](CCC(N([H])[H])=O)N([H])C(CN([H])C([C@H](CCC(O)=O)N([H])C([C@H](CC(C)C)N([H])C([C@H](CC(C=C4)=CC=C4O)N([H])C([C@H](CO)N([H])C([C@H](CO)N([H])C([C@H](C(C)C)N([H])C([C@H](CC(O)=O)N([H])C([C@H](CO)N([H])C([C@H]([C@@H](C)O)N([H])C([C@H](CC5=CC=CC=C5)N([H])C([C@H]([C@@H](C)O)N([H])C(CN([H])C([C@H](CCC(O)=O)N([H])C([C@H](C)N([H])C([C@H](CC6=CN=CN6[H])N([H])[H])=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)N([H])[C@H](C(N([H])[C@H](C(N([H])CC(N([H])[C@H](C(N([H])CC(O)=O)=O)CCCN([H])/C(N([H])[H])=N/[H])=O)=O)CCCN([H])/C(N([H])[H])=N/[H])=O)C(C)C
Solubility Chemicals:
Freely soluble in aqueous base (>pH7). Soluble in methanol (20mg/ml) and slightly soluble in DMSO or ethanol (1mg/ml).
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men: H. Agerso, et al.; Diabetologia 45, 195 (2002) | The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice: B. Rolin, et al.; Am. J. Physiol. Endocrinol. Metab. 283, E745 (2002) | The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits beta-cell apoptosis in vitro: S. Bregenholt, et al.; BBRC 330, 577 (2005) | Pharmacokinetics and pharmacodynamics of liraglutide, a long-acting, potent glucagon-like peptide-1 analog: J. Meece; Pharmacotherapy 29, 33S (2009) (Review) | The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease: P.L. McClean, et al.; J. Neurosci. 31, 6587 (2011) | Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection: C. Hoelscher; CNS Drugs 26, 871 (2012) (Review) | Liraglutide ameliorates glycometabolism and insulin resistance through the upregulation of GLUT4 in diabetic KKAy mice: L.N. Chen, et al.; Int. J. Mol. Med. 32, 892 (2013) | Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer's disease: P.L. McClean & C. Hoelscher; Neuropharmacol. 76, 57 (2014) | Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism: N.M. Krasner, et al.; PLoS One 9, e97554 (2014) | Liraglutide acutely suppresses glucagon, lipolysis and ketogenesis in type 1 diabetes: M. Garg, et al.; Diabetes Obes. Metab. 19, 1306 (2017) | Liraglutide, a glucagon-like peptide-1 analog, induce autophagy and senescence in HepG2 cells: G.C. Krause, et al.; Eur. J. Pharmacol. 809, 32 (2017) | Liraglutide inhibits the proliferation and promotes the apoptosis of MCF-7 human breast cancer cells through downregulation of microRNA-27a expression: W. Zhao, et al.; Mol. Med. Rep. 17, 5202 (2018) | Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells: A. Chen, et al.; BBRC 499, 267 (2018) | Liraglutide Modulates Appetite and Body Weight Via GLP-1R-Expressing Glutamatergic Neurons: J.M. Adams, et al.; Diabetes (Epub ahead of print) (2018) | Liraglutide exerts a bone-protective effect in ovariectomized rats with streptozotocin-induced diabetes by inhibiting osteoclastogenesis: B. Wen, et al.; Exp. Ther. Med. 15, 5077 (2018)