Rotenone
Product Code:
AG-CN2-0516
AG-CN2-0516
Regulatory Status:
RUO
RUO
Target Species:
Universal
Universal
Shipping:
AMBIENT
AMBIENT
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CN2-0516-G001 | 1 g | £55.00 |
Quantity:
| AG-CN2-0516-G005 | 5 g | £108.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Nicouline; NSC 8505; NSC 26258; Tubatoxin
Appearance:
White to off-white solid.
CAS:
83-79-4
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06,GHS09
Handling Advice:
Keep cool and dry.Protect from light.
Hazards:
H301, H317, H319, H335, H410
InChi:
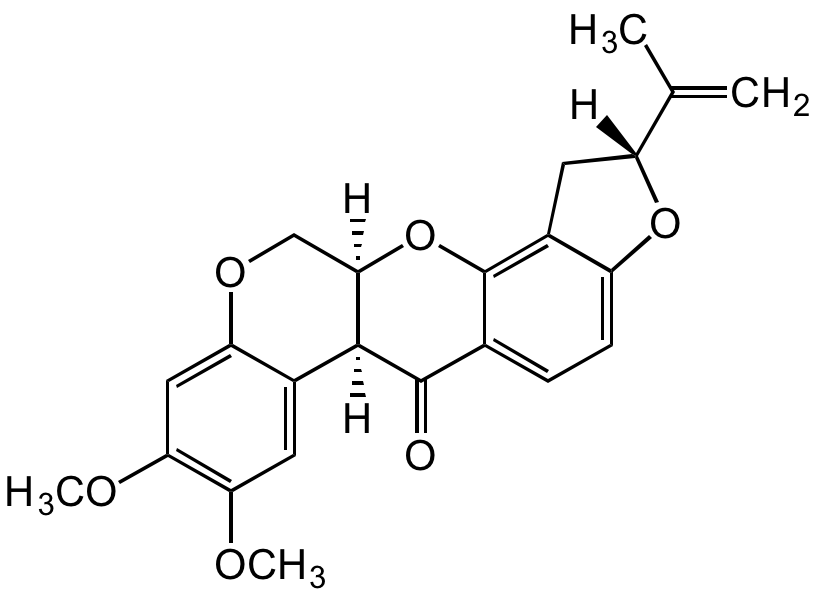
InChI=1S/C23H22O6/c1-11(2)16-8-14-15(28-16)6-5-12-22(24)21-13-7-18(25-3)19(26-4)9-17(13)27-10-20(21)29-23(12)14/h5-7,9,16,20-21H,1,8,10H2,2-4H3/t16-,20-,21+/m1/s1
InChiKey:
JUVIOZPCNVVQFO-HBGVWJBISA-N
Long Description:
Chemical. CAS: 83-79-4. Formula: C23H22O6. MW: 394.4. Synthetic. Originally isolated from Lonchocarpus nicou. Cell-permeable reversible and competitive mitochondrial electron transport chain complex I (NADH-CoQ reductase) inhibitor (IC50=1.7-2.2µM). Inhibits NADH/DB oxidoreductase and NADH oxidase and consequently oxidative phosphorylation (OXPHOS). Specifically inhibits NAD-linked substrate oxidation of NADH dehydrogenase. Useful agent for immunometabolism research. Inhibition of electron transport chain in mitochondria leads to blocking of the transfer of electrons from iron-sulfur centers in complex I to ubiquinone. This interferes with NADH during the creation of usable cellular energy (ATP), and Complex I is unable to pass off its electron to CoQ, creating a back-up of electrons within the mitochondrial matrix. Cellular oxygen is reduced to the radical, creating reactive oxygen species, which can damage DNA and other components of the mitochondria. Shown to inhibit mammalian cell proliferation, via suppressing microtubule assembly by binding to tubulin and inhibiting autophagy induction, by blocking lysosomal degradation of autophagic vacuoles. Shown to induce cell cycle arrest and apoptosis through production of mitochondrial ROS, consequently leading to induction of oxidative stress. Used to induce Parkinson's diseases-like syndrome in experimental animal model. Selective priming signal for NLRP3 inflammasome activation in combination with ATP, but not with Nigericin or MSU. Commonly used as a broad spectrum insecticide, piscicide and pesticide.
MDL:
MFCD09025614
Molecular Formula:
C23H22O6
Molecular Weight:
394.4
Package Type:
Vial
PG:
III
Precautions:
P261, P273, P280, P301+310, P302+352, P304+340, P305+351+338, P312, P330, P333+313, P337+313, P391, P405
Product Description:
Cell permeable reversible and competitive mitochondrial electron transport chain complex I (NADH-CoQ reductase) inhibitor (IC50=1.7-2.2µM). Inhibits NADH/DB oxidoreductase and NADH oxidase and consequently oxidative phosphorylation (OXPHOS). Specifically inhibits NAD-linked substrate oxidation of NADH dehydrogenase. Useful agent for immunometabolism research. Inhibition of electron transport chain in mitochondria leads to blocking of the transfer of electrons from iron-sulfur centers in complex I to ubiquinone. This interferes with NADH during the creation of usable cellular energy (ATP), and Complex I is unable to pass off its electron to CoQ, creating a back-up of electrons within the mitochondrial matrix. Cellular oxygen is reduced to the radical, creating reactive oxygen species, which can damage DNA and other components of the mitochondria. Shown to inhibit mammalian cell proliferation, via suppressing microtubule assembly by binding to tubulin and inhibiting autophagy induction, by blocking lysosomal degradation of autophagic vacuoles. Shown to induce cell cycle arrest and apoptosis through production of mitochondrial ROS, consequently leading to induction of oxidative stress. Used to induce Parkinson's diseases-like syndrome in experimental animal model. Selective priming signal for NLRP3 inflammasome activation in combination with ATP, but not with Nigericin or MSU. Commonly used as a broad spectrum insecticide, piscicide and pesticide.
Purity:
>97%
Signal word:
Danger
SMILES:
CC([C@@]1([H])CC2=C(O[C@]3([H])COC4=CC(OC)=C(OC)C=C4[C@]3([H])C5=O)C5=CC=C2O1)=C
Solubility Chemicals:
Soluble in DMSO (20mg/ml), ethanol (1mg/ml) or chloroform (20mg/ml).
Source / Host:
Isolated from Fabaceae species.
Transportation:
Excepted Quantity
UN Nummer:
2811
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Rotenone, an anticarcinogen, inhibits cellular proliferation but not peroxisome proliferation in mouse liver: M.L. Cunningham, et al.; Cancer Lett. 95, 93 (1995) | Rotenone-induced G2/M cell cycle arrest and apoptosis in a human B lymphoma cell line PW: J.S. Armstrong, et al.; BBRC 289, 973 (2001) | Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production: N. Li, et al.; J. Biol. Chem. 278, 8516 (2003) | Rotenone induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration: K. Radad, et al.; Neurochem. Int. 49, 379 (2006) | Rotenone inhibits mammalian cell proliferation by inhibiting microtubule assembly through tubulin binding: P. Srivastava & D. Panda; FEBS J. 274, 4788 (2007) | Pesticides and impairment of mitochondrial function in relation with the parkinsonian syndrome: C. Gomez, et al.; Front. Biosci. 12, 1079 (2007) | Mechanisms of rotenone-induced proteasome inhibition: A.P. Chou, et al.; Neurotoxicol. 31, 367 (2010) | Rotenone inhibits autophagic flux prior to inducing cell death: B.J. Mader, et al.; ACS Chem. Neurosci. 3, 1063 (2012) | Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson's disease models: N. Xiong, et al.; Crit. Rev. Toxicol. 42, 613 (2012) (Review) | Rotenone-induced oxidative stress and apoptosis in human liver HepG2 cells: M.A. Siddiqui, et al.; Mol. Cell Biochem. 384, 59 (2013) | The rotenone model of Parkinson's disease: genes, environment and mitochondria: J.T. Greenamyre, et al.; Parkinsonism Relat. Disord. 9, S59 (2013) (Review) | Metabolome and proteome profiling of complex I deficiency induced by rotenone: I. Gielisch, & D. Meierhofer ; J. Proteome Res. 14, 224 (2015) | Rotenone-induced Impairment of Mitochondrial Electron Transport Chain Confers a Selective Priming Signal for NLRP3 Inflammasome Activation: J.H. Won, et al.; J. Biol. Chem. 290, 27425 (2015) | Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages: E.L. Mills, et al.; Cell 167, 457 (2016) | A guide to immunometabolism for immunologists: L.A. O'Neill, et al.; Nat. Rev. Immunol. 16, 553 (2016) | The respiratory chain inhibitor rotenone affects peroxisomal dynamics via its microtubule-destabilising activity: J.B. Passmore, et al.; Histochem. Cell Biol. 148, 331 (2017)